METHANE
Page 21
Page 22

Page 23

If you've noticed an error in this article please click here to report it so we can fix it.
The Motor Fuel
of England Technical Facts about SaturatedHydro carbons which Include Petrol and Methane. There are Vast Sources of the Latter Which is an Attractive Motor Fuel
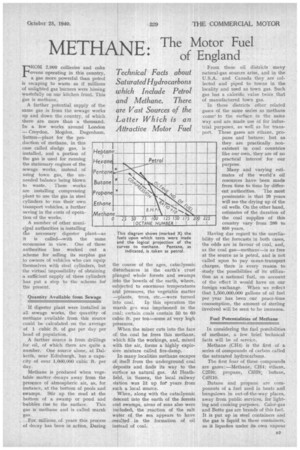
Heptane Hexan Pentane
.Butane Propan .Ethane Methane FROM ROM 2,000 collieries and coke ovens operating in this country, a gas more powerful than petrol is escaping to waste as if millions of unlighted gas burners were hissing wastefully on our kitchen front. This gas is methane.
. A further potential supply of the same gas is from the sewage works up and dawn the country, of which there are more than a thousand. In a few works around London — Croydon, IVIogden, Dagenhana, Sutton—plant for the production of methane, in this case called sludge gas, is • installed, and a portion of the gas is used for running the stationary engines of the sewage works, instead of using town gas, the un • needed balance being blown to waste. These works are installing compressing
• plant to use the gas in steel cylinders torun their own transport vehicles, a further saving in the costs of operation of the works.
A number of other municipal authorities is installing the necessary . digester pla it—as it is called—with the same economies in view. One of these authorities has worked out a ,scheme for selling its surplus gas to owners of vehicles who can equip themselves with steel cylinders, but -the virtual impassibility of obtaining , st sufficient supply of these cylinders has put a stop to the scheme for the present.
. .
Quantity Avallab e from Sewage If digester plant were installed in all sewage works, the quantity of methane available from this source could be calculated on the average of 1 cubic ft. of gas per day per head of population.
, A further source is from drillings for oil, of which there are quite a number. One source alone, at Dal • keith, near Edinburgh, has a capacity of over 1,000,000 cubic ft. per day.
Methane is produced when vegetable matter decays away from the presence of atmospheric air, as, for instance, at the bottom of pools and swamps. Stir up the mud at the bottom of a swamp or pond and
-bubbles rise to the surface. This gas is methane and is called marsh gas.
For. millionssof years this process of decay has been in action. During
the course of the ages, cataclysmic disturbances in the earth's crust plunged whole forests and swamps into the bowels of the earth, where, subjected to enormous temperatures and pressures, the vegetable matter • —plants, trees, etc.—were turned into coal. In this operation the marsh gss was imprisoned in the coal; certain coals contain 50 to BO cubic ft. per ton—some at very high pressures.
When the miner cuts into the face of the coal he frees this methane, which fills the workings, and, mixed with the air, forms a highly explo sive mixture called fire-damp.
In many localities methane escapes of itself from the underground coal deposits and finds its way to the surface as natural gas. At Heathfield, in Sussex, the local railway station was lit up fat years from such a local source.
When, along with the cataclysmic descent into the earth of the forests and swamps, areas of seas also were included, the -reaction of the salt water of the sea appears to have
resulted in the formation of oil instead .of coal. From these oil districts many natural-gas sources arise, and in the U.S.A. and Canada they are collected and piped to towns in the locality and used as town gas. Such gas has a calorific value twice that of manufactured town gas.
In these districts other related gases of the same series as methane come* to the surface in the same way and are made use of for industrial purposes, as well as for transport. These gases are ethane, propane and butane; but as they are practically nonexistent in coal countries like our own, they are of no practieat interest for our purpose.
Many and varying estimates of the world's oil resources have been made from time to time by different authorities. The most pessimistic is that 20 years will see the drying up of the oil wells. On the other hand, estimates of the duration of
225 the coal supplies of this country vary from 200 to 850 years.
Having due regard to the unreliability of the forecasts in both cases, the odds are in favour of coal, and, as the coal gas—methane--is as free at the source as is petrol, and is not called upon to pay ocean-transport charges, there is every reason to ,study the possibilities of its utilization as a national fuel, on account of the effect it would have on our foreign exchange. When we reflect • that 1,500,000,000 gallons of oil fuel per year has been our peace-time consumption, the amount of sterling involved will be seen to be immense_
Fuel Potentialities of Methane
In considering the fuel possibilities of methane, a few brief technical facts will be of service.
Methane (CH4) is the first of a series of compounds of carbon called the saturated hydrocarbons.
The first four of these compounds are gases :—Methane, CH4; ethane, C2H6; propane, C31-18; butane, C4I-110.
, Butane and propane are components of a fuel used in boats and bungalows in out-of-the-way places, away from public services, for lighting and cooking purposes. Calor-gas and Botto gas are brands of this fuel. It is put up in steel cOnta,iners and the gas is liquid in these containers, as it liquefies under its own vapour pressure when the bottles are closed up.
These gases, along with ethane, are the products of the fractional distillation of crude oil, and are therefore imported fuels and outside our consideration.
The next five compounds of this series cover the various qualities of motor spirit, generally called petrol, although this name was registered by one of the original suppliers of this liquid fuel (Carless, Capel and Leonard).
Pentane C51-11, Various _ Hexane C.H„ combinations Heptane C7H in different
Oetane C„H,„ .qualities of Nonane C,,H„ petrol Range of Distilling Points
. The distilling points of these compounds range. between 40 degrees C. and 150 degrees C.
. Beyond these, again, are further liquids, which include paraffin and lubricating oils. Beyond, again, up to CoH,„ are solid compounds, including vaseline, the paraffin wax and asphalts.
• Methane is a gas at ordinary temperatures and pressures. Its boiling point is —164 degrees C., and it solidifies at --184 degrees C. By the application of pressure, the boiling point can be raised to —82 degrees C., called the critical point, above which point it cannot exist as a liquid. The pressure required at this point is 45 atmospheres.
The 'density of the liquid is just over half that of petrol. The calorific value is 22,500 B.Th.U.s per lb. —petrol being 19,500. The air/ gas mixture is 9.5 to 1, compared with 13 to 1 for petrol.
• 4 Measure of the' Fuel Economy
The calorific value of the explosive mixture is not the indication of the power to be obtained from the fuel, but of the, fuel economy. .
The power available is a function of the highest possible compression ratio, which, again, is dependent Upon the octane number of the fuel'. So little is known of the properties 'of methane that the octane number is still Shrouded in mystery. It is given by various authorities as being 'from 110 to 125.
'Much light is thrown on the pos'sibilities of this point by. a study of the general properties of the saturated hydrocarbon 'compounds, some of which are given in an accompanying chart.
It will be noted how the properties of the Various compounds follow 'with great regularity the sequence of the number of carbon atoms in the . compound,. Another point for remark • • is that the hydragen'atcim is always
twice that of the carbon atom, plus 2. It is logical to assume that otherproperties will also follow this regularity.
Opposite is a chart of engine tests on .various fuels, giving b.h.p., compression ratio of engine, and octane number of the fuel used, made by the Philgas Corporation of America on a six-cylindered engine of 4-in. bore by 5-in, stroke.. This con1PallY makes and distributes the liquefied' gases propane and butane, -which are largely used in America. for transport
and industrial power and heating purposes.
It ' will be noted that, with the appropriate compression ratio, 'butane gives 26 per cent, more power than' petrol, 'whilst propane gives over 50 per cent. more.
" Following the chart, and projecting back to methane, gives us some of the probabilities of that Nei with regard to eventual power values throbgh the medium of compression .ratios and octane values.
The values so arrived at appear to be very 'high, and they are, of course, subject to actual experimental research, but they point the way to the possibilities, which may be modified in practice, but we have :alwaks the figures already, attained on butane and propane 'as a guide and landmark, as 'it were, on the road to methane.
.
Methane is usable in existing engines without even increasing the compression ratio, inasmuch as town gas is now being used under such conditions, with the addition only of a gas mixer instead of the existing carburetter; alternatively, the petrol carburetter can be retained, so that either fuel can be used. Private cars have been so converted, so as to run on Calor gas, and without increasing the compression ratio,' are equal in performance to, petrol. An engine test made , on Calor gas,
with the compression raised. from 6.25 to 8 to 1, showed an increase -of 12i per cent. in b.h.p.
• Engines with 'compressions of 14 to. 1 or 15_ to 1 lend themselves to the possibilities • of methane. Although it will be necessary in most cases to install electric ignition, a combination' of oil and methane, using 5 per cent. of oil, could' be employed with compression-ignition. This is, however, using imported fuel for the ignition, _unless gas oil, home produced , by our town-gas works—which is suitable for the purpose—can be produced in .sufficient 'quantities to obviate the• use of imported Oils.
Looking farther ahead, when gas -engines can be designed to use methane_ as a gas—which it is, and which petrol is not—the considerable increase in power available, as shcwn earlier in this article, will lead to smaller engines being made to do the same work as the present larger ones.
An indication of this possibility is that an 8 h.p. car would require at most only a 6 h.p engine—and possibly smaller—to do the same work, when final values are worked out by the necessary experimental processes.
Liquefaction of Methane It is only a makeshift to attempt to run transport on fuel which has to be carried as a gas; so little can be stored as to forbid running reasonable distances before refuelling, whilst the plant required to compress the gas, and the cylinders to carry it on the vehicle make it quite impracticable as a possible permanent solution of the problem. Further, as an immediate alternative, it is also impracticable, as the compressing plant and alloy-steel cylinders are practically unobtainable owing to war conditions.
The alternative is to liquefy the gas, when the transport and storage on the vehicle are such that reasonable distances can be covered before refuelling. It is quite a practical possibility to liquefy methane; it was done experimentally 50 years ago. It has again been done quite recently and an 8 h.p car has been successfully run on the liquefied gas.
• A commercial project is nearing completion for the liquefaction of the gas, and its transport from source to user. This project necessitates consideration of the temperatures and • pressures given earlier in this article.
The Philgas Company in America has commercially worked out the necessary plant, which has been in operation for 15 years, for the storage and transport of the related liquefied gases, butane and propane. The relationship between these gases and methane is shown in the following table :— Degrees C.
Boiling Melting Point. Point.
Methane C.H.4 —1.64 —186 Ethane C2H.6 —93 —172 Propane C3H.6 —45 — 152 Butane C4H.10 —0.1 -135
The problem is therefore one of degree, and it has been tackled and solved both in Germany and in Italy where this gas is used quite extensively for transport purposes: in Gerrr any it is known as " Motoren Methane," and, in Italy, " Metano."
Steam .Vehicles It may well he that methane will have an effect on the possibilities of utilizing steam for road transport.
In conjunction with the lias11 boiler, and high steam pressures— up to 1,000 or 1,250 lb. per sq. in.— and superheats up to 700 degrees C., the possibility of accurate control of a gas-fired boiler are greatly superior to any system using 'a liquid fuel.
Consideration is being given to these possibilities in certain interested quarters. AzorE.




















































