THE HYDROGEls ALTERNATIVE
Page 66

Page 67
If you've noticed an error in this article please click here to report it so we can fix it.
The abundant natural sources of this element plus its ability to burn cleanly in oxygen makes it an attractive alternative fuel, but Allan Winn finds that it has some heavy drawbacks
ALMOST front the moment that the first internal combustion engines were tired up. manufacturers and custonit:rs have been looking for a fuel in limitless supply. It was recognised that world resources of economically-recoverable tOssil fuels were limited, and that alternatives to internal combustion — such as steam and electricity — were unlikely to prove viable. The most obvious alternative to petrolemn-based fuels is alcohol.
Alcohol can he obtained from the fermentation of plants, which are a replaceable resource, making it a replaceable fuel. Unformnately, although Brazil, especially, has made progress with alcohol fuels, the sheer size of crop needed to provide it in reasonable quantities limits the technology to countries with huge areas of suitable farmland and a good climate.
Another fuel which seems to have a great attraction, at least at first sight, is hydrogen. Hydrogen is a superabundant element, present in huge numbers of naturally-occurring compounds of which the most common is water, 1-1,0. It can be easily obtained front fossil fuels, and Cal) also be obtained from water through electrolysis.
I lydrogen has several other advantages apart from its widespread occurrence in nature. One of the most appealing is that it burns in oxygen to form water: if it could be burned in pure oxygen, it would be the cleanest fuel available. Obviously, if hydrogen is burned in air, all sorts of by-products conic into the equation, so that the oxides of nitrogen (NON) which bedevil the use of fossil fuels at high combustion temperatures, for instance, become a probh.sni 11ydrogen also has a rather unfortunate tendency to explode when large concentrations of it are ignited. 'Hie public horror of spectacles such as the destruction of the hydrogen-tilled
airship ifiudenbew makes it all too clear why tanks of hydrogen stored under pressure on motor vehicles would be completely unacceptable in the market. hi the long term, however, hydrogen remains one of the very few alternative fuels with a genuine chance of practicability. At the moment it is largely a possibility rather than a practicability, but its potential use (if rather limited) has been demonstrated in the last few years, especially by Daimler-lienz.
The successful use of hydrogen as a fuel depends largely on its safe storage and transportation. The most practical way of attaining this appears to be to store the hydrogen in a chemical compound from which it can be easily extracted. It is the metal hydrides which are the prel-erred compounds for this: they are compounds of metal alloys and hydrogen. The alloys used normally contain metals such as iron, magnesium, titanium, manganese, nickel or chromium.
Introducing hydr igen to these alloys causes an exotherm'c reaction, in which the hydrogen is absorbed (to Corm the hydride) and heat is given off. The sa amount of heat (either stored, or extracted from the exhaust gases of th running engine) is needed to extract t hydrogen from the hydride when it is required.
While this is a clean and safe way o storing hydrogen, it has the rather telling drawback that the metal hydric is not light, and nor is the tank which required to hold it. (Me tank needed the alternative method of using hydrogen — as liquid hydrogen storec under extreme pressure and inside massive thermal insulation — is also great, but not as great as this.) For one of the more attractive lowtemperature hydrides using an iron/titanium alloy, the weight of hydride needed to store hydrogen wit the equivalent calorific value when burned as a litre of normal petrol is around 15kg. The tank structure need, to contain this hydride works out at another 10-12kg for each litre-equivah for a practical size of tank. So a tank containing as much hydrogen as woul he the equivalent of 30 litres of petrol would weigh around 800kg.
While such weights may appear .cessive when compared with the lessan-50kg for the equivalent petrol and tank, they are rather less than the .uivalent in batteries to do the same b with electrics. Using the best iction batteries currently available, hich store 3(1 watt-hours/kg, a vehicle mild need five tonnes of batteries to 3re the energy contained in those 30 res of petrol.
The use of liquid hydrogen has not sen ruled out. While the structure vded to contain the equivalent of a set )Iume of petrol as hydrogen is greater, .d weighs more than the petrol tank. rdrogen is itself very light. It is rather Lergy-inefficient to convert hydrogen its liquid state, and rapid conversion .eds the assistance of a catalyst. The big problem with liquid fdrogen is, however, that it tends to
• aporate, and considerable amounts ill be lost in normal storage. The large nks used for liquid hydrogen storage the moment suffer to some extent, ough losses are usually under one per nt a day. Apart from the inefficiency 'that, there is the added hazard of idrogen drifting around inside a thick or garage. Such leakage has to be iannelled straight to the engine, vented asitively to atmosphere or converted dug catalysts to water.
Few modifications are required to .ake a normal spark-ignition engine run hydrogen as a fuel. One important ctor is that hydrogen-burning engines nd to produce more NOx in their exhaust gases than do equivalent petrol engines. The measures taken to counter this can be either recirculating the exhaust gases through to the engine, or injecting water into the cylinders: water injection seems to be the most successful.
Another problem is that hydrogenburning engines tend to back-fire or suffer from pre-ignition. •Ihese effects can also be countered by injecting water into the cylinders, or by keeping the hydrogen out of the engine until the ideal time for ignition is reached. That means injecting the hydrogen under pressure — as is done in the normal diesel engine — after the inlet valve has closed.
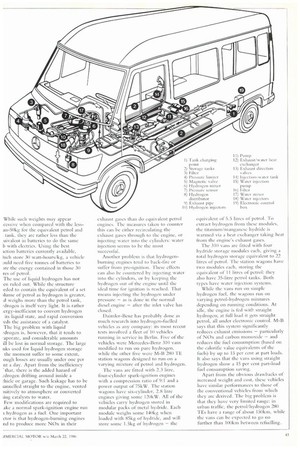
Dainder-Benz has probably done as much research into hydrogen-fuelled vehicles as any company: its most recent tests involved a fleet of 10 vehicles running in service in Berlin. Five of the vehicles were Mercedes-Benz 310 vans modified to run on pure hydrogen, while the other five were M-B 280 'FE station wagons designed to run on a varying mixture of petrol and hydrogen.
The vans are fitted with 2.3 litre, four-cylinder spark-ignition engines with a compression ratio of 9:1 and a power output of 75kW. The station wagons have six-cylinder, 2.8 litre engines giving sonic 120kW. All of the vehicles carry hydrogen stored in modular packs of metal hydride. Each module weighs some 140kg when loaded with 85kg of hydride, and will store some 1.5kg of hydrogen — the equivalent of 5.5 litres of petrol. To extract hydrogen from these modules, the titanium/manganese hydride is warmed via a heat exchanger taking heat from the engine's exhaust gases.
The 310 vans are fitted with four hydride storage modules each, giving a total hydrogen storage equivalent to 22 litres of petrol. The station wagons have two modules each, storing the equivalent of 11 litres of petrol: they also have 35-litre petrol tanks. Both types have water injection systems.
While the vans run on simple hydrogen fuel, the wagons run on varying petrol-hydrogen mixtures depending on running conditions. At idle, the engine is fed with straight hydrogen; at full load it gets straight petrol, all under electronic control. M-B says that this system significantly reduces exhaust emissions — particularly of NOx and carbon monoxide — and reduces the fuel consumption (based on the calorific value equivalents of the fuels) by up to 15 per cent at part loads. It also says that the vans using straight hydrogen show a 15 per cent part-load fuel consumption saving.
Apart from the obvious drawbacks of increased weight and cost, these vehicles have similar performances to those of the conventional vehicles from which they are derived. The big problem is that they have very limited range: in urban traffic, the petrol/hydrogen 280 TEs have a range of about 150km, while the vans can be expected to go no further than 100km between refuelling.






















































































