UPKEEP OF COMMERCIAL ELECTRIC VEHICLES
Page 16

Page 17
If you've noticed an error in this article please click here to report it so we can fix it.
The Fifth Article.
THE BATTERIES USED for driving vehicles are of the secondary type ; they require that an electric; current shall be passed through them for a certain number of hours, before they will furnish current to the electric motor driving.the vehicle. They are sometimes called electric accumulators or storage batteries, because they store the electric energy furnished by the current, and deliver a certain portion of it again when called upon. There are two forms of secondary battery upon the market—the lead-oxidesulphuric acid battery, and the iron-nickel-caustic potash battery.
The Battery.
The lead battery has been known and experience gained with it for over 30 years ; the iron battery, or the alkali battery as it is more usually termed, has only been on the market for the last few years, though Mr. Edison and others had been experimenting upon it for nearly 30 years. Few men have had very much experience with the alkali • battery ; those who have are principally on the staffs of the Edison Co.
In the lead battery, there are an edd 'number of lead grids, held in outer containing vessels of glass, celluloid, or teak lined with lead • strong glass is the best form of containing vessel, because the working of the cells can be seen more or less through the glass. For electric vehicles, a certain number of cells are placed in a wood box or trough, arranged to be easily lifted in and out of the space provided for it under, or on, the vehicle.
The larger Fig. 25.—A positive plate and a number of grids negative plate from a cell disposed are negative corparallel to each other and connected responding to the to opposite lead bars—positive and negative. plates
-TERM BAR + PLATE —PLATE
negative respectively. of a primary bat tery, the smaller number being positive. Thus, with 11 grids, 6 are negative and 5 are positive; this is BO that both sides of the positive grid can be faced by a negative grid. When the grids are in the cell, the positives are held between the negatives, as is shown in Figs. 25 and 26; all the positives are connected together by a lead bar as shown in the figures, and all the negatives by another lead bar. When a battery is ma.cle up, the negative bar of one cell is connected to the positive of the next cell, the negative of that cell to the positive of the next, and so on, a positive bar being left free at one end, and a negative bar at the other end, as shown in Fig. 26. The bars of adjacent cells are connected together by special forms of terminal screws, or clamps. One of the most important things inconnection with the working of secondary batteries is to see that all the connecting screws or clamps are making good contact. They should. be examined every time the battery is recharged. This applies also to the end terminal screws, from which cables are taken to the controller, etc. If a terminal screw, or the wire that is connected to it, is slack, sparking will very probably take place there, leading later to a disconnection ; and, in any case, a resistance will be inserted at the point where the loose contact is, the current which!should pass
out from the battery or into it being lessened in consequence.
The lead grids carry oxides of lead ; the negatives carry a low oxide-, the positives-a high oxide ; and when a charging current flows through a cell, the high oxide on the positives is raised to a higher oxide, and the low oxide on the negatives is reduced to pure lead in the spongy form.
The electrolyte in the lead battery is dilute sulphuric acid; its specific gravity before charge is from 1115 to 1170; after the •are charge it is 1275, and it may go up to 1300. The charging current, in addition to changing the oxides, also in-creases the density of the electrolyte ; and a test of this density forms a very good guide for the condition of the charge..
The lead grids are merely the carriers for the active material. They convey the charging currents to the active material and to the electrolyte, and they convey the current during dieeharge from the active material to the outer circuit, the controller, the motor, etc.
Lead cells have a pressure of 2 volts per cell, when fully charged. Immediately after charging they may have a pressure as high as 2.2 volts, but it falls very quickly to 2 volts, and it continues to fall, whether current is taken from the battery or not, from the moment the charging current ceases to flow. Fig. 27 shows curves of the fall of pressure of the average lead cells, when discharged in different periods. All secondary batteries work better when discharged at a low rate ; but modern batteries keep well up to their work when discharged at higher rates, providing that they are promptly recharged. It is of great importance that the cells should not be discharged so that their pressure falls below 1.75 volts per cell, and they work better if they are not discharged below 1.8 volts, and better still if they are kept to 1.85 volts. During discharge, the specific gravity of the electrolyte falls to anywhere between 1115 and 1170, according to the pressure to lvhich they are discharged and the rate of aischarge.
When they are being charged the lead cells require a pressure of from 2.2 volts per cell up to 2.75 volts. Fig. 28 shows a charging curve for an ordinary lead' cell, at the 10-hour rate ; it will be noticed that for a large portion of the charge the Pressure is about 2.2
÷ PLATES 'PLATES
Fig. 26.—A pair of cells showing the connection between the positive plates of one cell with the negative plates of the next.
volts per cell; it is only towards the end of the charge that the pressure rises, first to to volts, and then right at the end of the charge, when gassing takes place, to 2.75. The reason of the higher pressure required in the charging current is that each cell, when being charged, develops a back pressure of from 2.2 volts upwards, and the charging current requires to have sufficient pressure to overcome this back pressureand to force the charging current through the cell. The pressure is higher at the end of the charge, because a portion of the electrolyte is decomposed into its component gases, and this is what is meant by
gassing. In central station batteries, gassing is usually allowed to take place, but, for vehicle batteries, it is wiser to stop the charging current at the first sign of gassing. Every time gassing takes place the electrolyte loses a small portion of its liquid, and that has to be made up again. It is of the utmost importance that the make-up should be of pure distilled water and, when acid is required, only pure "brimstone " acid should be used, not that made from pyrites. Serious trouble arises if impurities are allowed to get into the battery, either in the acid or in the water. Small local actions are set up which tend to lower the efficiency of the battery and to neutralize a portion of the active material.
Sulphating: How It Arises and How It Is Cured
If a lead battery be allowed to be discharged below the pressure mentioned above, or if it be allowed to stand idle for any time, what is called sulphating is set up, sulphate of lead being foimed on the lead grids between them and the active material. Sulphate of lead is practically an insulator, considered from the point of view of the pressures employed in lead secondary batteries' and so the formation of any of it breaks the connection between the lead grid where it is formed and the active material, reducing the possible output of the battery. The charging current does not-reach the oxide where the sulphate has been formed nor does the discharging currentpass through it. In addition, there is always a tendency for the sulphate to spread, once it has commenced to form. It is known by its appearance, a dead white colour. The remedy is careful attention to the charging, careful testing by voltmeter and hydrometer as every' opportunity. Every cell should be tested at the end of each run for pressure and sp. gr., and at the end of each charge, and a keen look-out should be kept for the appearance of any sulphate. When sulphate is forming on the lead grids, it appears on the surfaces of the grids where there is no oxide usually, as well as between the oxide and the grids. When a cell tests badly at the end of a charge, eelphating should be suspected and the cell removed, carefully examined and recharged. If a cell has been badly sulphated, the best plan is to charge it and discharge it several times, carefully testing it each time. If it does not build up, a reverse charge (the current flowing through it in the opposite direction to that usual for charging) may be tried, this being followed by a charging current. When a cell
is badly sulphated, or if it has been allowed to fall much below 1.75 volts, it is wise to make the charging current considerably weaker than is usual and to continue it for a longer time. Electro-chemical actions are always better when carried out slowly.
What has to be done to give a badly-sulphated battery new life is to convert all the sulphate into oxide, and this is best accouiplished by a .comparatively weak current flowing for a comparatively long time. These remarks apply to any cases where sulphate has formed or is suspected, and the building up should be followed very closely by the voltmeter and the hydrometer. It should be mentioned, however, that every time sulphate of lead is formed arid is converted into oxide, something is taken from the substance of the lead grids_ The grids may be looked upon as the bones of the battery and the sulphate eats into them just as some diseases eat into human bones. If much sulphating takes place, the grids become so weakened' that they are apt to fall to pieces.
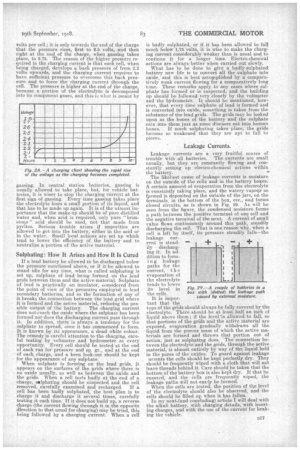
Leakage Currents.
Leakage currents are a very fruitful source of trauble with all batteries. The currents are small usually, but they are constantly flow,ing and constantly setting up electro-chemical actions within the battery. The likeliest cause of leakage currents is moisture on the outside o.f the cells and in the battery boxes. A certain amount of evaporation from the electrolyte is constantly taking place, and the watery va.potir so fprmed is deposited on the outs:de of the jars, on the terminals, in the bottom of the box, etc., and forms closed circuits, as is shown in Fig. 29. As will be seen from the figure, the condensed moisture forms a path between the positive terminal of one cell and the negative terminal of the next. A current of small value flows continuously around this path, steadily discharging the cell. That is one reason why, when a cell is left by itself, its pressure steadily falls—the leakage cur
rent is stead
ily dischargr
evaporation of ing it. In addition to forming leakage paths for the current, t h e It is important that the
tops of the grids should always he fully covered by the electrolyte. There snoutd be at least half an inch of liquid above them ; if the level is allowed to fall, so that portions of the grids and. the active material are exposed, evaporation gradually withdraws all the liquid from the porous mass of which the active materials are formed and throws that portion out of action, just as sulphating does. The connection between the electrolyte and the grids, through the active material, is almost entirely by way of the liquid held in the pores of the oxides. To guard against leakage urrents the cells should be kept perfectly dry. They should be frequently wiped with a cloth that will not leave-threads behind it. Care should.be taken that the bottom of the battery box is also kept dry. If that be ensured, and the cells are frequently wiped, • the leakage paths will not easily be formed.
When the cells are tested, the position of the level of the electrolyte should also be observed, and the cells should be filled up when it has fallen.
In my next.(and concluding) article I will deal with the alkali battery, with charging details, with boosting charges, and with the use of the current for braking the vehicle.






















