You can't top this battery
Page 49

Page 50
If you've noticed an error in this article please click here to report it so we can fix it.
EARLY THIS YEAR A. C. Delco made its Freedom battery, with a claimed design life of 300,000 miles, available in the UK. This is the first lead calcium completely maintenance-free vehicle battery to be launched on the European market.
Maintenance free means exactly that; once fitted it never needs the electrolyte level to be topped up, and in fact, there is no facility to do so. The terminals and cable ends are made of special stainless steel resistant to corrosion — so again, once connected they should never need to be looked at.
The advantages to the vehicle operator are in cost savings on maintenance and reliability as it dispenses with vehicle breakdown owing to the failure of a neglected battery.
Two major American battery companies, AC Delco and Gould, started development work independently back in 1 960 both looking for materials for the casing and the internal plates which would give them the fit-and-forget characteristics they wanted.
This year all General Motors' vehicles fresh off the production line will include the new Delco battery in the standard specification.
Gould, on the other hand, without the benefit of Delco's connections with a major vehicle manufacturer, has had to content itself competing in the replacement market.
Both batteries use lead calcium plates, a very efficient conductor of electricity — in itself is no great new technological break-through. Lead calcium plates have been used in the construction of large stand-by batteries in use by telephone companies for more than 20 years. Their most important attribute was that they were able to hold a high level of charge for long periods.
Unfortunately when the plates were produced in a much reduced size to suit the vehicle application they lacked the strength to support themselves.
Overcharging
In the conventional battery antimony is added to the lead to give it the necessary strength and rigidity. However, by overcoming this problem in this way reduces the ability of the plate to hold its charge for long periods. Once charged, antimony starts to transfer from the positive to the negative plate causing a partial separation of the electrolyte into hydrogen and oxygen gases, which once allowed to pass through the breather vents are responsible for terminal corrosion.
Antimony has also been blamed for the battery's limited resistance to overcharging, another cause of gassing. In older vehicles the performance of the voltage regulator is often less efficient than it ought to be. Once the plate becomes dry it may suffer damage which will not be put right by the replenishment of water.
Reduced water loss
All that needed to be accomplished to produce a non-maintenance battery was to stop, or dramatically reduce, the water loss. Lead calcium seemed to provide the basis of a solution.
Delco developed a new manufacturing process in which wrought lead calcium alloy is rolled to give it a fine granular structure. These are stronger than cast grids, very uniform in texture and highly resistant to corrosion.
In the assembly of the battery the plates are _ positioned low down in the casing allowing about twice the volume of electrolyte above the elements, but no space below to allow sediment to settle out.
Calcium defects
Calcium is not without its own defects. When it comes into contact with oxygen a reaction takes place to form calcium oxide which gives the lead calcium plate a tendency to flake. If allowed to settle out it could lead to premature failure.
In place of conventional separators special envelopes were developed to. contain the • plates yet allow them to function without a reduction in performance.
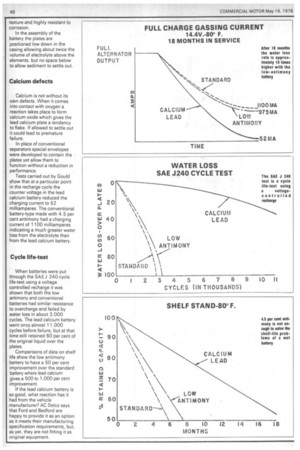
Tests carried out by Gould show that at a particular point in the recharge cycle the counter voltage in the lead calcium battery reduced the charging current to 52 milliamperes, The conventional battery-type made with 4.5 per cent antimony had a charging current of 1100 milliamperes indicating a much greater water loss from the electrolyte than from the lead calcium battery.
Cycle life-test
When batteries were put through the SAE ...I 240 cycle life-test using a voltage controlled recharge it was shown that both the low antimony and conventional batteries had similar resistance to overcharge and failed by water loss in about 3,000 cycles. The lead calcium battery went onto almost 11.000 cycles before failure, but at that time still retained 60 per cent of the original liquid over the plates.
Comparisons of data on shelf life show the low antimony -battery to have a 50 per cent improvement over the standard battery where lead calcium gives a 500 to 1,000 per cent improvement.
If the lead calcium battery is so good, what reaction has it had from the vehicle manufacturer? AC Delco says that Ford and Bedford are happy to provide it as an option as it meets their manufacturing specification requirements, but, as yet, they are not fitting it as original equipment.












































































































