FORD VAN POINTERS.
Page 26
Page 27

If you've noticed an error in this article please click here to report it so we can fix it.
By R. T. Nicholson (Author of "The Book of the Ford ").
AB I said at the end of my remarks of last week when I opened the subject of the battery, there is the big difference between the bell battery and the accumulator (or storage battery) such as is employed in the lighting system of a Ford vehicle.
459 Contd.—The Action of the Accumulator.
The chemical action of the electrolyte in the bell battery gradually disappears ; you restore it by adding more sal-ammoniac. The zinc is gradually eaten up by the electrolyte ; you have to supply new zincs from time to time. The accumulator behaves in the same way as regards interaction of electrolyte and elements ; that is why the battery gets exhausted ; but you can restore the electrolyte and the " elements " to active condition by passing back an electric current through the accumulator. That is what is called charging. The charging is going on all the time your generator is running ; so the accumulator is kept constantly charged, notwithstanding its being also frequently partially discharged for lighting and starting.
The fact is that discharge of the battery produces certain chemical changes in it, which changes are undone by passing a current back through the battery. I need not go into the nature of those changes at present. I merely want you at this point to get hold of the facts that :—
(1) The output of electricity is due to the different chemical action of the electrolyte on the two elements.
(2) That this action is undone by passage of an electric-current through the accumulator. You cannot see into the Exide battery (as you can into batteries made with celluloid containers); but if you could, you would see in each cell a number of brown plates and a number of grey ones. The former correspond with the sine rod in the bell battery, and the latter with the carbon slab. Technically, the former are called the positive and the latter the negative plates. (The positive " element " is that which is more vigorously attacked by the electrolyte.) You would find that when the battery was well charged the brown i plates would be a deep chocolate colour, and that they would get perceptibly paler as the battery discharged. This change of colour shows that chemical action is at work on the plates.
The Chemical Action.
(Do not worry too much over this paragraph, although it may help you to understand what goes on in the battery.)
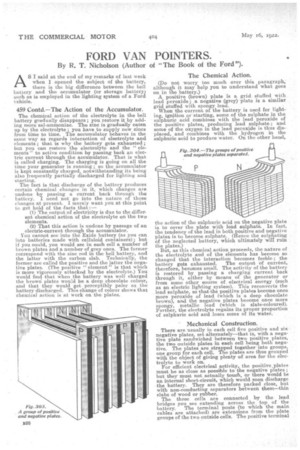
A positive (brown) plate is a grid stuffed with lead peroxide; a negative (grey) plate is a similar grid stuffed with spongy lead.
When the current of the battery is used for lighting, ignition or starting, some of the sulphate in the sulphuric acid combines with the lead peroxide of the positive plates, producing lead sulphate ; and some of the oxygen in. the lead peroxide is thus displaced, and combines with the hydrogen in the sulphuric acid to produce water. On the other hand, the action of the sulphuric acid on the negative plate is to cover the plate with lead sulphate. In fact, the tendency of the lead in both positive and negative plates is to become sulphate. (Hence the sulphating of the neglected battery, which ultimately will ruin the plates.) But, as this chemical action proceeds, the nature of the electrolyte and of the elements has become so changed that the interaction becomes feeble ; the battery gets exhausted. The output of current, therefore, becomes small. The activity of the battery is restored by passing a charging current hack through it, either by means of the generator or from some other source of electrical energy (such as an electric lighting system). This reconverts the lead sulphate, so that the positive plates become once more peroxide of lead (which is a deep chocolate brown), and the negative plates become once more spongy, metallic lead (which is slate-coloured). Further, the electrolyte regains its proper proportion of sulphuric acid and loses some of its water.
Mechanical Construction.
There are usually in each cell five positive and six negative plates, set alternately—that is, with a negative plate sandwiched between two positive plates, the two outside plates in each cell being both negative. The plates are strapped together into groups, one group for each cell. The plates are thus grouped with the object of giving plenty of area for the electrolyte to work on. For efficient electrical activity, the positive.plates must be as close as possible to the negative plates ; but they must not actually touch, or there would be an internal short-circuit, which would soon discharge the battery. They are therefore packed close, but with non-conducting separators between them—thin slabs of wood or rubber. The three cells are connected by the lead bridges you see extending across the top of the battery. The terminal posts (to which the main cables are attached) are extensions from the plate groups of the two outside cells. The positive terminal post (that from which the fat cable runs forward from the battery) is an extension of a positive group of plates, and the negative terminal post (that from which the fat cable runs to " earth ") is an extension of a negative group of plates.
What You Should Now Know.
You should now have considerable respect for your battery. You should now know that it must be treated respectfully—that it must have proper attention.
You now know what battery discharge means. It means that the electrolyte has become watery. This means that it can no longer properly attack the "elements." You know, too, why the hydrometer teat tella you of the condition of the battery at any time, if the electrolyte h a s become watery (by discharge of the electricity, due to chemical action), it will not be s "dense" — i t s specific gravity will get low, simply because
sulphuric acid weighs more than water. You should see why it will not do for you to raise the specific gravity by adding more acid—because, if you do that, you will not assist the plates to get rid of their sulphate when you next have to charge your battery.
Perhaps I ought here to tell you why you must use distilled water in "topping the electrolyte— in bringing it up to its proper level. It is mainly because much rainwater contains impurities which, for one reason •or another, would upset the action of the sulphuric acid on the plates. It may, for instance, contain iron ; and this iron would get de
Fig. 305. —Sectional view of battery, showing sediment space at bottom. posited on the plates and make a series of small batteries the action of which would upset the action of the battery as a whole. Or the water may be slightly alkaline, and that, again, would interfere with the proper action of the sulphuric acid. Oh viously, dirty water taken from a well or a, pond or from a rainwater tub might contain all sorts of impurities, and throw the chemical interaction between the electrolyte and the "elements" right out of truth.
Why Batteries Wear Out,
One reason why batteries wear out is that the " paste " with -which the grids of the plates ai e packed tends to fall out, lodging in the spaces pro vided to receive it at the bottom of each cell.. (If it did not lodge there it would do mischief elsewhere.) This loss of paste weakens the action of the battery. By the way, never wash out the battery with the idea of removing the " sludge " thus produced, ii you do, you will shift it to some point where it will do much harm, such as will certainly arise if you get bits of the positive plate jammed against a. negative plate. The reason for this shedding of paste is manifold. The effect is partly due to road vibration, partly to electrical violence, and partly to, the " scrubbing " action of the electrolyte that goes on when your generator is trying to charge a battery which is already fully charged, and can "take no more." Ideally, we ought to have some means of disconnecting the generator when the battery is fully charged. As, however, the paste has got to escape from the grids of the plates any way, it cannot be said that, overcharging is a great evil.
With hard use, the violence of electrical discharge may buckle the plates and damage the separators.
The positive plates shed more material-than the negative plates. In average use, it is well to replace the positive plates once every two years, and the negative plates once every four years. In practice, this means that it is well to replace the whole battery at the end of every fourth year, the positive plates having been replaced at the end of the second.
I do not pretend to have told you in the foregoing how you should treat your battery and what regular attention it needs. I have dealt with these practical questions fully before, and I shall doubtless do so again. Here I have only tried to show you main principles—why proper treatment is needed, net what that treatment is in detail.
































