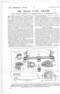
ABSORPTION X PRESSURE.
Page 16

Page 17

If you've noticed an error in this article please click here to report it so we can fix it.
An Interesting Development in Coal-gas Storage for Vehicles.
AST WEEK, in our Editorial pages, we commented upon the _opportunity presented to in ' ventive genius in the field of applying coal-gas as a fuel te, heavy-vehicle transportation. That the diligent workers in the laboratory are fully alive to the possibilities in this realm, and are energetioally pursuing active investigation to ease the crisis in the motoring world, is being revealed to us from every side. The
problem revolves around the devising of ways and means to increase radius of action upon' a single fuel charge, without any disproportionate augmentation of weight, so as to bring coal-gas more into line with petrol. The problem admittedly bristles with complexity, but the issue is being appreciably eased at the moment from the inventor's point of view by relegating the weight factor to a secondary position. . During the past few -days we have been afforded the opportunity te investigate the latest development in this direotion. At the moment it has not proceeded beyond the laboratory stage, but it has been advanced to such a point as to demand application to practical -trial, to determine its commercial possibilities, arrangements for conducting which, we understand. have been completed.
A New Line of Investigation.
The invention is based upon the property of certain substances to -absorb gases, in much the same way-as blotting paper will lap up ink. It is the work of two collalaorateurs, the one a B.Sc. with honours in chemistry, -20 years experience in the laboratory, including considerable research in pure ecience, and an intimate acquaintance with chemical problems as they affect coal-mining, coal-fuels and gases ; the other, a private practical motorist since the early days of the petrol vehicle. The circtuestance that the idea has been thrashed out by practical minds should be sufficient to arouse more than usual interest, especially as the co-workers fully realize the significance of commercial practicability. •
After investigation of the generally-exploited methods of storing coal-gas, -both at free OT low pressure, and under compression, the chemist decided to strike out upon a line. This, so far as we have been able to ascertain, has never previously been followed up seriously with coal-gas, although the fundamental principles in the most elementary form have been known for at least 50 years.
The list of gases, both commercial and otherwise, is lengthy. Now each of these gazes•possesses a decided fancy or affinity for one or more materials, and is capable of being readily absorbed by the media, the character of which varies considerably. This knowledge has been put to commercial account in certain directions, as, for instance, in connection with acetylene producing what is colloquially—though
errotrously—known as dissolved acetylene.
Selecting the• Absorbents.
The character of these absorbents is by no means secret to the select few. Anyone who is anxious to familiarize himself with their nature has only to search the files of the records of scientific achievement during the last half-century. The list is somewhat imposing, and, accordingly, the first step was to sift out the most suitable for dealing with the gaseous mixture of variable composition known as coal,. or town, gas. This proved one of the most tedious of many preliminaries, involving many experiments as well as close consideration of the relative costs and ready availability of the respective substances. The exigencies of commercialism had to e51 be borne in mind, since the question of price is ever the determining factor of the practicability of such an invention.
The experiments in the laboratory speedily proved fruitful. But not only were interesting results recorded : the field of investigation became appreciably enlarged. The coal-gas storage question became re-, solved into another and peculiar problem—the storage and transport.of energy in small bulk and weight.
So far as this particular problem is concerned, the question turned upon_ the discovery and selection of two absorptive materials. The constituents of coalga-s may be resolved into two broad groups—methane 60 per cent., and hydrogen 40 per cent. respectively.
Consequently, onemedium was required for the former and another for the latter. This precipitated a peculiar issue. Whereas the determination of the methane absorbent was relatively simple, the range of suitable substances being from 40 to 60, and fluctuating in price, the possible hydrogen absorbents Were few in number and vastly different in character. A particle of this absorbent is so sin-all that no mechanical appliance capable of grinding it to the necessary degree of fineness is known. Yet it is capable of absorbing hydrogen to the extent of many times its own volume.
The characteristic of the hydrogen absorbent seemed likely to provoke an insurmountable obstacle. Being so exceedingly fine, rather in the form of a quasi vapour, it proved difficult to control. Upon releasing the valve of the container, naturally the absorbent would be hurled into the outer air by the agitation, and so would be lost, thus necessitating recharging with hydrogen absorbent with every replenisliMent—a hopelessly iinpracticable proceeding.
How Hydrogen is Harnessed.
Thereupon the chemist set to work to render the hydrogen absorbent more stable, to bring it uncle' complete control, and at the same time prevent its escape with the out-rushing gas. This has beer accomplished by taking the minute particle of hydro gen absorbent and encasing it within a particle of ths methane absorbent. This imprisoning of one absorbent within the other, and in such a way as tc form a homogeneous unit, constitutes an achievement of chemical science and the crux -of the inven tion.
Once the harnessing of the hydrogen absorber was -achieved, progress was rapid. A further surve2 of the available methane absorbents revealed th( ability to meet the needs of every class of motorist In other words, the cost of the container could lai adapted to meet various pockets. Thus it is possibi. to equip a container upon this principle for les. th. methane absorbent in this instance being a materia which at present is regarded purely as a waste pre duct. On the other hand, a material costing as mud] as -£14 per ounce may be used for the self-same pin Pose.
The reason for grading the absorbents may be ea plained by analogy. It is well known that blottin paper varies in ink-absorptive capacity or .efficien-ce A sheet 12 ins. square, costing the tenth part of penny, will not absorb so much as a sheet of ,egus size costing ten times at much. It is the same wit these gas absorbents. Consequently, the motoris who acquires an expensive absorbent reaps the ac vantage from higher first cost in enhanced radiusc action per charge, dimensions and weight of containe remaining constant.
The advantages of greater absorptive capacity an efficiency are fully brought out when resort is mad to compression in addition to absorption, because in this way it is possible to accentuate the absorptive capaeity of the !material. This can be explained very readily. We will aesume that the lower-grade absorbent has an absorbing factor of 5 per CUJAC foot at atmospheric pressure. Taking a cylinder measuring 48 ins. in length by 7 ins. diameter (internally), we find that its cubical capacity is a little in excess of cubic foot, which figure will suffice for our illustration. If, therefore, a cubic foot of absorbent will absorb 5 feet, cubic foot will receive 2 cubic feet at atmospheric pressure.
Such a cylinder as that described above is built to receive oxygen, hydrogen and acetylene in the free state up to a pressure of 120 atmospheres. We will take the lower figure of 100 for our purpose. Thus we get 4 x 100 = 250 cubic feet, which represents the volume of gas which the absorbent within a cylinder of cubic foot capacity can be induced to absorb when pressure up to 1400 lb. perk square inch is applied. By resorting to a material having a higher absorbing capacity at atmospheric pressure, and then resorting to compression, it is possible to induce the absorbent to take up a proportionately greater volume of the gases. At the moment the inventors are able to prepare absorbers possessing up to 100 times the volume of the cheapest grade at atmospheric pressure. Taking an absorbent having an efficiency at atmospheric pressure of 50, which i3 10 times that used in our previous example, and taking a pressure of 50 atmospheres, we now get 50 x 50 = 2500 cubic feet contained within a cylinder of cubic foot capacity. The advantage is obvious : the petrol equivalent is raised from 1 gallon in the first instance to 10 gallons in the second case, without any increase in the dimensions of the container or its weight, merely by baying recourse to an absorbent of higher efficiency and compression. Thus it will be seen the first cost may be speedily recouped.
The action of the absorbents may be briefly described. Upon coming into contact therewith, the coal-gas is split into its two broad gas groups— methane and hydrogen—each to be seized by its individual absorbing 'medium. The hydeogen absorbent being imprisoned within the methane absorbent, naturally the hydrogen has to pass through the latter, while a certain proportion of this gas becomes absorbed by the 'methane ,absorbent, and a certain per
centage of the methane by the hydrogen absorbents In breaking up the coal-gas and absorbing the twd constituents the proportion is broadly maintained,, so that, upon release, the gases recombine in did correct percentages to reconstitute the original gas.
What is the weight of a container necessary to fulfil these requirements? Upon this cardinal point little satisfaction can be vouchsafed at the moment, seeing that the inventors are. compelled to be content with • what commerce is able to supply them under present conditions. This is the issue which is certain to receive immediate attention upon the cessation of war, and which ia equally certaih to popularize the employment of coal-gas as a motor fuel. Fortunately, owing to the acute character of the petrol problem, the weight question is not the vital issue which certain detractors would wish us to believe. In the present instance the familiar cylinders used for storing oxygen, acetylene and other commercial gases are being used. The total weight thereof, including absorbents, is approssimately 130 lb., but it must be remembered that, in this case, owing, to the varying. quantity of gas which -can be compressed and absorbed within a given cubic capacity, weight loses significance. It is the volume of gas which constii tutes the governing factor. One distinct advantage accrues from the use of contemporary cylinders. While the absorbents are indestructible and permanent, their efficiency. is exposed to a slight degree of depreciation due to impurities in the coal-gas, moisture and other causes. The cylinders in question have to be subjected to re-annealing every six months, and this process . serves to revivify the absorbents, and td restore to them their original capacity and efficiency.
Charging-up is conducted in the usual way. It is recommended that the gasiehould be free from moles ture before passing through the compressor; but this is a simple and inexpensive procedure, merely.necessitating passing the gas over -calcium chlonde, a. cheap and readily-obtainable material.
We are informed that a series of tests with coalgas fuel storage in this manner are to be conducted upon the high road within the near future. We hope to have the opportunity to witness these trials, to satisfy ourselves of the-possibilities, of the invention, ana to determine the extent of its commercial application.