Lubricants and Lubrication for Motor Vehicles.
Page 2

Page 3

If you've noticed an error in this article please click here to report it so we can fix it.
The Relative Merits of High-Grade and Low-Grade Lubricating Oils. A Case for Good Quality and Purity.
During the early days of the motor movement, the efficient lubrication of the engines and the transmission gearing of the vehicles as they were then made was an expensive and serious matter. The cost of the lubricants which each wagon consumed " in the course of a day's run became so serious that the very existence of more than one carrying concern was endangered. The amount of oil actually used in lubricating the various parts of a wagon was very small, when compared with the large quantity that was distributed along the roads, and which often left a trail that marked the progress of the machine. At that time, the splash system of lubrication was practically universal, and the amount of oil that leaked through the crankshaft bearings, engine casing, and other channels, due to the presence of too large a quantity in the crankcase, was, undoubtedly, the cause of the regrettable demand for the cheap, or rather low-priced, lubricants which are now so much in evidence. The object of the present article is to point out to users of commercial vehicles that real economy can be effected by the use of high-grade oils, and, in a future article, we hope to show how such lubricants can be most economically and effectively applied in typical cases.
The use of the splash system undoubtedly leads— on ac.. count of the uncertainty which exists in the mind of a driver as to the state of the oil level within the crankcase— to the excessive use of oil, which is " consumed " in a variety of ways. Part of it goes for the legitimate purpose of maintaining a very thin film of oil between the pistons and the cylinder walls, or between the journals and their respective bearings; a portion of it finds its way past the pistons, and is carried off by the exhaust gases in the form of heavy and offensive vapour; an uncertain quantity of it escapes past the end bearings of the crankshaft and camshafts, or through the valve-tappet guides; and a part of it is drained off from the crankcase as being too foul for further use as a lubricant for the engine.
The motor vehicle of to-day is, in point of cleanliness, a long way in advance of the wagons which competed in the Liverpool trials of ISO and subsequent years, and, although there is still a large amount of oil lost through the engine and other bearings, this percentage is, by reason ef the improvements in design which are constantly being made, daily becoming less than that which was experienced by the pioneers of commercial motoring. A member of the Society of Road Traction Engineers, in a recent contribution to " THE COMMERCIAL MOTOR " (see the issue for the 28th Max' last) outlined a scheme of lubrication by which he had obtained remarkable results with several public-service motorbuses. Those results show that, with such a low rate of consumption of oil, the relative cost of the lubricant is a comparatively unimportant matter, and that the user should, lo use the words of Professor Thurston in his book " Friction and lost work in machinery and mill work," purchase all lubricants " with a careful regard to their value, rather than by reference to their price : their value is determined principally by their friction-reducing power and their reduction of wear of rubbing parts."
Let us first consider the nature of the various oils and fats which are available, and the sources from which they are obtained. All oils and fats can be divided into two classes : the hydrocarbon variety, or mineral oils; and the glycerol, or fatty animal and vegetable oils.
Hydrocarbon Oils.
Oils of this class are obtained from the shales of Scotland, France, and New South Wales, or from the petroleum wells and springs of America, Russia, Germany, Burma, and other countries. They may, roughly, be divided into four groups, viz :—(I) limpid and colourless liquids, known as naphtha, benzoline and benzine, which are very volatile, and have specific gravities which range from o.68 to 0.76: (2) water-white, limpid liquids known as parallel, petroleum and kerosene, the specific gravities of which vary from o.78 to o.85 ; (3) yellow oils whose specific gravities range from o.85 to 0.915, and which are more or less viscid, anti therefore suitable for use as lubricants for machinery ; and (4) viscous oils whose colours vary from dark amber to deep brown. All the above bodies are found to be more or less complex mixtures of naphthenes, paraffins, and olefins. The crude petroleum from which the above oils are obtained is an oily liquid with a most offensive smell, and the colour of which varies from yellow to dark brown. It consists of at least thirty hydrocarbons, and these are separated out into various groups by a process of fractional distillation ; as each fraction is collected, it is purified and washed with sulphuric acid and caustic soda. The heavy residue that is left in the still, after the benzine and kerosene have been evaporated, is still further treated, and from it lubricating oils, vaseline, and paraffin wax are produced.
Animal and Vegetable Oils.
Olive, Rape-seed, Linseed, and Cotton-seed oils are obtained from the fruit, or seeds, of the plants named, by the application of pressure, or by extraction of the oils with the aid of some volatile solvent, such as petroleum spirit; animal oils are obtained by a process of rendering the fatty portions of the flesh of certain animals. All the oils and fats in this series contain acids, and some of these, such as oleic acid and stearic acid, are common to many of the oils and fats in this class, whilst others, such as arachidic acid, ricinoleic acid, and linolic acid, are found in only a few. With the exception of the ricinoleic series, all these acids are soluble in petroleum spirit, whilst all are soluble in alcohol, ether, and benzine; oils which contain these acids are, therefore, markedly unsuitable as lubricants for internalcombustion engines, on account of the action which the acids are likely to have upon the cylinder walls and other parts of the engine, whilst their use as lubricants for any other form of machine should be restricted to the crudest forms of construction.
Compound Oils.
A very large percentage of the oil which is now used for lubricating purposes belongs neither to one nor the other of the two distinct classes to which we have referred. Such oils are combinations, in varying proportions, of a hydrocarbon oil with one or other of the fatty oils, and, although in some cases their use may be tolerated, they are not to he recommended for use on motor vehicles, either for the lubrication of cylinders or any other part of such a machine. The ordinary methods of distillation were, no doubt, perfectly satisfactory for the purpose for which they were introduced, namely, the production of cheap burning oils, but, during the process of distillation by those methods, the heaviest constituents of the oil undergo certain changes, and are partly decomposed, and their residues in the still contain a quantity of tarry deposits. Many oil refiners attempt to utilise these residues for lubricating purposes, but, although most of them are highly viscous, they arc absolutely unsuitable for the purpose. It is true that, on account of its high viscosity, such a residue may prevent the metal of a journal from coming into actual contact with its bear. ing, but, on account of the stickiness of the fluid, the relative movement of the journal and its bearing is resisted to such an extent that a measurable amount of work has to he expended in the overcoming of the friction of the socalled lubricant. In order, therefore, to reduce the stickiness, or rather to make it less easily detected, a few refiners compound the residue with an animal or vegetable oil, and offer the resulting compound as "the thing " for motor lubrication, In fairness, however, to at least one refiner who advocates the use of compound oils, we would add that the practice of using up the residues in this manner is not universal, and that, with some compound oils, the hydrocarbon is practically free from tarry deposit before its compounding with the fatty oil. The acids which are associated with the fatty oils are, however, always present in compounded oils; at high temperatures, these adds are liberated, and they can then attack the surfaces of the metal.
Viscosity and Flash Point.
The state at which an oil will pass through a given aperture is called specific viscosity, and by many men high viscosity is regarded as a sure indication that the oil is suitable for use for a given purpose; this property, however, is not the only thing to be taken into account when choosing an oil; it may, to all intents and purposes, be viscous, as we have stated to be the case with some of the tarry residues in the stills, and yet be unsuited for lubricating purposes. It is the "oiliness" that is of prime importance, although, speakinggenerally, the heavier the pressure and the slower the speed of a journal, the higher should be the viscosity of the oil. When considering this questiOn, the first thing to be taken into account is that heat reduces the viscosity; tests have shown that the body of an oil, when at a temperaure of 212 degrees Fahrenheit, is only about three to ten per cent, of its body at 70 degrees Fahrenheit, whilst at 300 degrees Fahrenheit it is only about two-and-a-half to six per cent. of that at 70 degrees Fahrenheit. ,
It is not our intention to discuss such questions as the temperature of the flYrite within the combustion chamber of an engine, or whether the flame ever touches the cylinder walls, but we think we are right in assuming that the mean temperature of the cylinder walls is about 350 degrees Fahrenheit, and the lubricating oil for use in such a cylinder should, therefore, have a flash-point of goo degrees Fahrenheit, or even higher ; at that temperature, the oil should resist all tendency to dissociation or decomposition, and the depositing of gummy and tarry substances.
Convincing Experiments.
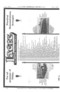
For the purpose of comparing some of the oils which are now on the market, we wrote, on the sth of May last, to all the leading refiners and dealers, and requested that samples of their oils should be forwarded to us; as a result of those applications, we received 32 samples of oil, and many samples of thick grease. The latter material has not been considered in our argument, which has been directed more particularly to the problem of engine lubrication, as it is in this direction that one must look for the econotnising of oil ; greases form a much less important item in the bill of costs for lubricants. Having obtained the samples, we proceeded to submit them to various chemical tests, with the object of detecting the presence of any acids, gums, tarry deposits, carbon deposits, or other undesirable eonstituents, and the broad results of our tests are set out in the accompanying table. A measured quantity of each
sample of oil was taken, and to it was given a number. By this number only was the sample known to the gentleman who conducted the experiments, and no questions as to the maker of any particular sample were entertained until the tests were completed. We refrain, for obvious reasons, from publishing names, but a close study of the results that are given in our table will prove instructive to many of our readers. It will be seen that only six out of the 32 samples were quite free from both acids and deposits; these oils remained perfectly fluid throughout the tests, and their oiliness was not in any way impaired by the action of the acids with which, for the purposes of the experiments, they were mixed. These six arc Nos. 1, 9, 15, 16, 17, and 20. Four Of the compound oils, Nos II, 12, 73, and 19, showed no signs of deposit, but there were unmistakable signs of the presence of acid. The other " observations " speak for themselves.
Conclusions.
After careful consideration of all the results of the experiments, we are driven to the following conclusions i. The best-quality, hydrocarbon oils are absolutely necessary, or there will be some evil influence at work, slowly eating away the surfaces of the metal, or gumming up the parts, until their impaired condition involves extra work in order to overcome the increased friction.
2. Inferior oils lead to sparking troubles, due to the excessive quantity which is required to perform a given duty; they are decomposed by high temperatures; and they leave deposits on the pistons and other parts, thus necessitating more frequent overhaul and cleaning. 3. The best oils, such as those which are indicated in the accompanying table by the Nos. 15 and 16, do not require to be drained off from the crankcase like other oils, because they do not carbonise or leave any deposit at high temperatures. This lasting quality is certainly a step in the direction of economy, and, owing to the small quantities that will serve for the efficient lubrication of all parts of the engine, the smoke nuisance is, so far as it is attributable to the oil, avoided. 4. The use of a really good oil, now that constructional detail has practically eliminated leakages, effects a real economy, even if its price is four times that which one would pay for some of the oils which are enumerated in the table.
5. Engine builders commonly advise owners never to use less than one gallon for each 200 miles on the road for a four-cylinder engine, but with a good oil, and a proper method of applying it, the same quantity can be safely made to last for four times that distance in commercialvehicle work.