(From the Orewinq Bor
Page 32

Page 33
If you've noticed an error in this article please click here to report it so we can fix it.
e
by Graham Montgomer e IN THIS, the first article in a regular fortnightly series, Graham Montgomerie looks at recent developments in turbocharging.
IN RECENT years, the science of exhaust gas turbocharging has progressed in leaps and bounds. It is an attractive proposition to the design engineer, as by sensible matching of the turbocharger to the engine he can obtain higher power, increased torque, better specific fuel consumption and lower combustion induced engine noise.
This of course presumes that the basic engine structure is strong enough to cope with the increased performance — but that's another story.
In the world of medicine, many of the drugs used to treat the patients have undesirable side effects, and this analogy can be applied to the turbocharger. An undesirable side effect, alongside the many benefits, is a very high combustion temperature which is the major contributing factor to the oxides of nitrogen in the exhaust gas.
Increasing pressure from government legislation has forced the engine manufacturers to look at ways of decreasing this NOx with a two-tier system in the USA. As yet there is no legislation for this in Europe but the factories are anticipating this to come in the mid-80s.
First principles
When air is compressed by the turbocharger, its temperature is raised considerably. This is not due merely to the air's proximity to the hot exhaust turbine — the main temperature rise is due to the adiabatic compression of the air at the compressor stage of the turbocharger. If this compression process was 100 per cent efficient, then in thermodynamic terms this rise in temperature could be calculated thus:
where T1 = temperature of intake air to compressor P2 = static pressure ratio across the compressor
Pi stage K=1.395 thus K-1=0.283 Putting some numbers in, a typical pressure ratio of 2,4 to 1 and an air intake temperature of 27 C (81 F) would give a theoretical temperature rise of 84 C (183' F). However, centrifugal compressors of the size would in typical commercial vehicle turbochargers have an isentropic efficiency of the order of 72 per cent, so the actual temperature rise over the compressor is nearer to 117 •C (243 F). This means that the temperature of the engine intake air as it leaves the turbocharger is now around 144 • C (291 F).
As the power produced by an engine is proportional to the mass of air drawn in, then the inlet temperature is a critical factor as the higher the temperature, the lower the density for a given volume.
Charge-cooling or, aftercooling or inter-cooling (call it what you will) is increasingly being applied by many manufacturers to reduce the temperature of the high pressure inlet air before it enters the cylinder thus increasing its density and allowing increased fuelling.
Manufacturers who now produce charge-cooled turbocharged engines include Cummins, Rolls-Royce, Perkins, DAF, Volvo, Berliet, Mack and Caterpillar. Indeed the NEC was a good place to see several of these a few weeks ago.
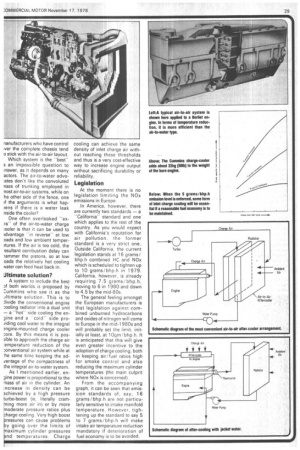
Pros and cons Two systems of chargecooling are in current use: airto-air, whereby the heat from the inlet air is transferred. to the ambient air by means of an auxiliary radiator, and air-towater where the unwanted heat is removed by water in the same manner as the engine cooling system.
The air-to-air system is favoured by the "vehiclebuilders, ie, Berliet Volvo, OAF, etc, whereas the "engine" builders prefer the air-to-water technique. The odd one out is Perkins, who as proprietary engine builders, prefer the air-to-air system.
There are pros and cons for both systems but one remarli which applies equally to both i! that the most important charac teristics of any charge coolinc system is its efficiency ir reducing the air temperature This efficiency can b( represented mathematically 13) the expression E — T4 — T3 x 100% T4 — Tc where T4 = temperature ol air into the charge cooler T3 = temperature ol air out of the charge cooler Tc = inlet temperature of the cooling medium (is air or water)
From this it can be seen that E 1 00 per cent efficient charge cooler will reduce the air tem perature to that of the coolinc medium. However, not man■ things in life are this efficien. and the charge cooler is no ex ception.
In practical terms, ar efficiency of 75 per cent is abou. the norm. Efficiencies highei than this can be achieved but a the expense of size and weigh. with the end result being C traditional engineer's compro. mise.
Taking a typical air-to-watei system, the practical reductior in air temperature is of the ordei of
AT = (T4 — Tc) X 0.75 = (144 — 90) x 0.7! = 40'• C (104. F)
By the use of ambient air as the cooling medium, a much better reduction can be achieved, eg.
AT = (144 — 27) x 0.75 = 88 C(190' F) The resulting improvement in air density is thus around 10 and 25 per cent respectively. If sheer efficiency was the sole criterion, then the case for air to-air would be overwhelming.
As far as the engine builders are concerned, however, they want to have control over the charge-cooler and for this reason Cummins and Rolls-Royce use an air-to-water systerr which is built into the inlei manifold as part of the overal engine package. Vehicle
nanufacturers who have control iver the complete chassis tend o stick with the air-to-air layout.
Which system is the "best" s an impossible question to inswer, as it depends on many actors. The air-to-water advo:ates don't like the convoluted naze of trunking employed in nost air-to-air systems, while on he other side of the fence, one the arguments is what hap)ens if there is a water leak nside the cooler?
One often overlooked "exra" of the air-to-water charge :ooler is that it can be used to sdvantage "in reverse" at low oads and low ambient temperstures. If the air is too cold, the .esultant combustion delay can -sammer the pistons, so at low oads the relatively hot cooling ivater can feed heat back in.
Ultimate solution?
A system to include the best af both worlds is proposed by Cummins who see it as the Jltimate solution. This is to divide the conventional engine cooling radiator into a dual unit — a "hot" side cooling the engine and a -cold" side providing cool water to the integral angine-mounted charge cooler core. By this means it is possible to approach the charge air :emperature reduction of the conventional air system while at :he same time keeping the advantage of the compactness of the integral air-to-water system.
As I mentioned earlier, engine power is proportional to the mass of air in the cylinder. An increase in density can be achieved by a high pressure turbo-boost (le, literally cramming more air in) or by more moderate pressure ratios plus charge cooling. Very high boost pressures can cause problems by going over the limits of maximum cylinder pressures and temperatures. Charge cooling can achieve the same density of inlet charge air without reaching these thresholds and thus is a very cost-effective way to increase engine output without sacrificing durability or reliability.
Legislation
At the moment there is no legislation limiting the NOx emissions in Europe.
In America, however, there are currently two standards — a -California" standard and one which applies to the rest of the country. As you would expect with California's reputation for air pollution, the former standard is a very strict one. Outside California, the current legislation stands at 16 grams/ bhp.h combined HC and NOx which is scheduled to tighten up to 10 grams/bhp.h in 1979. California, however, is already requiring 7.5 grams/ bhp.h, moving to 6 in 1 980 and down to 4.5 by the mid-80s.
The general feeling amongst the European manufacturers is that legislation against combined unburned hydrocarbons and oxides of nitrogen will come to Europe in the mid-1980s and will probably set the limit, initially at least, at lOgrn / bhp.h. It is anticipated that this will give even greater incentive to the adoption of charge cooling, both in keeping air/fuel ratios high for smoke control and also reducing the maximum cylinder temperatures (the main culprit where NOx is concerned).
From the accompanying graph, it can be seen that emission standards of, say, 16 grams/ bhp.h are not particularly sensitive to intake manifold temperature. However, tightening up the standard to say 5 to 7 grams/bhp.h will make intake air temperature reduction mandatory if deterioration of fuel economy is to be avoided.






























































































































