• TURNING NAPHTHALENE INTO MOTOR FUEL.
Page 12

Page 13
Page 14

If you've noticed an error in this article please click here to report it so we can fix it.
A Notable Invention Which Indicates a New DevelOpment in the Motoring Industry.
IN AN EDITORIAL articleo few weeks ago, we drew Attention to the prizes awaitmg. the inventor who successfully indicated ways and means of widening the range of home-produced motor fuels. Absence of proved petroleum deposits in these islands compels us to fall back upon the fruits which the distillation of coal is able to yield. This was the line of research we indicated as promising the greatest results, and we also emphasized the stimulus which contemporary conditions should impart to inventive fertility. We are now able. to draw attention to one achievement in this direction—one which we might mention gives every indication of great promise, owing' to the mere circumstance that the substance in question possesses qualities which have been proved. The invention has been carried through what may be described as the initial laboratory stage. It has been transferred from the retort to practical utilization— within limitations certainly, but this very restricted application under everyday working conditions has served to substantiate what the laboratory retort indicated. We do not go so far as to say that the invention has been brought' within the reach of commercial application: far from it. But we do think that the matter should now be taken up seriously inasmuch as success would now seem to be entirely dependent upon the perfeetionof details. The fuel is there with its properties incontrovertibly proved. Now it is merely a matter of evolution to turn the discovery to the greatest commercial advantage and highest efficiency.
What is Naphthalene ?
The fuel in question is one of the heavier hydro-carbons derived from the distillation of coal—naphthalene. It is familiar to every gas-engineer, much to his eternal disgust and chagrin, inasmuch as it is at the bottom of 00 per cent, of all the troubles between the gas consumer and the gas-producing company. It is not even a necessary evil which, consequently, calls for toleration. It is suffered in gas merely because its removal would incur a certain .expense,, possibly necessitate the substitution of another ,hyerocarbon to a limited degree, and because its reclamation would only swell the accumulation of a product which has ordinarily little commercial value. Apart from being the constituent of certain dyes and explosives, which, by the way, have been superseded by those in which benzole and toluol loom largely, naphthalene has no industrial use beyond forming the readily combustible conatituent of fire lighters, and, to a lesser degree, as a so-called disinfectant euphemistically though fallaciously labelled "carbon," and also as an,
i antidote to moths n the preservation of clothing. Its value in these two last-named directions is far naorei imaginary than real. It is, however, used as an antiseptic sometimes, but the quantity required for this purpose is small. ,
But for, years past it • has been maintained that naphthalene possessed virtues as a fuel. for internalcombustion engines. The problem has been to discover the scientific application of these properties. This reasoning is logical. Naphthalene. belongs to the benzoic group as much as paraffin wax belongs to the petroleum group. Moreover, it is not so very far removed from benzole in its chemical composition. Whereas the symbol of the first named is COIs that of naphthalene is Cell. It was this comparatively close relationship which stimulated inventors to research. But they pursued erroneous lines of investigation, as their patents and subsequent experiments have conclusively demonstrated.
. Thus, one group of inventors sought to use naphtha, lene as a constituent in a mixed fuel. Cognisant of the fact that benzole will absorb in solution about 40 eafi
per cent, of naphthalene, they followed this line of application and-then endeavoured to utilize the resultant liquid fuel in the usual manner. But such a, mixture is certain to provoke, endless trouble. The heavier hydrocarbon, when attempts are made to volatilize it in the usual manner with the ordinary spray carburetter, separates at the spray, and resumes its crystalline form in the choke tube and induction pipe, after the manner in which it condenses or precipitates in our gas mains and pipes. For this reason, the combined f eel fell into disfavour.
• Use as a Straight Fuel
The second group of inventors endeavoured to use straight naphthalene—that is the hydrocarbon in its unadulterated form. Here there was a subdivision of thought. The one class depended upon sdlid injection to achieve the desired end. The other class first liquefied it by the application of heat followed by vaporization with the familiar spray carburetter. But here, again, failure was encountered. Solid injection was uncertain as well as complicated:, Liquefaction and carburation provoked trouble unless the na-phthalene were very pure. The very finely divided•foreign raatter carried in suspension collected at the jets, thus choking them and rendering`thern inoperative. .
In the latest invention all these objections have been overcome. While success primarily depends upon raising the naphthalene to a highly-heated condition, it is not called upon to pass through a jet or any Other fine passage, nor does it have to be dissolved in a more volatile liquid, although it is possible for the last-name-d practice to be pursued if thought desirable. This invention is the product of two experimenters lately attached to the Corporation Gas Works of Birmingham—Mr. J. H. Willis, A.M.I.Mech.E., and Lieutenant G. G. Wilson, R. War. Regt.—both of whom have ,been associated with the higher branches of gas technics for many years, while at the moment Mr. Willis is closely identified with vital operations under the Ministry of Munitions. The invention has been tested upon a limited scale, which, had the season not been so far advanced before practical experiments could be taken in hand, would probably have been productive of more impressive results. A 25 ft. motor boat, fitted with a 10 h.p. four-cylinder engine of American construction, belonging to Mr. Willis, has been equipped to run on this fuel, and has put up some interesting runs exclusively upon it..
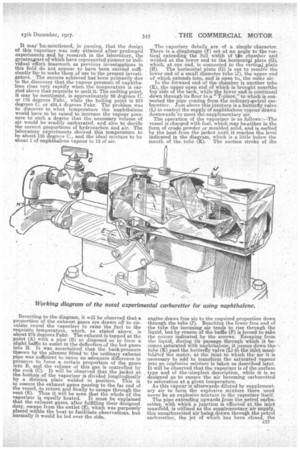
The invention is simple, and, being free from complicated mechanism, possesses the advantage of not being readily disorganized. It turns upon a special type of vaporizer which, in reality, is a combined fuel tank and surface carburetter. The general features of this device are shown in the aecompanying tion, which further indicates its installation 'upon the motor boat in question. It must be explained, however, that this model is purely experimental. The inventors have already designed a new model possessing many noteworthy improvements, but the basics principle remains the same.
Description of Vaporizer.
The -vaporizer comprises a rectangular tank surrounded at the bottom, ends, and sides with a iacket. This vessel is built up of thin steel plate with all joints welded under the oxy-acetylene process, inasmuch as it has to withstand great heat. In this particular instanee,athe inlet and exhaust manifolds of the engine are placed one above the ether, winch has somewhat facilitated the fitting of the vaporizer, but the apparatus can be applied with equal success to any type of engine. It mayTheimentioned, in passing, that the design of thia vaporizer was oniy attained afterprolonged experiments and by research in the laboratory, the greaterspart of which have represented pioneer or individual effort inasmuch as previous investigations in this field do not appear to have been carried sufficiently far to make them of use to the present investigators. The success achieved has been primarily due to the discovery that the vapour pressure of naphthalene rises very rapidly when the temperature is carried above that requisite to melt it. The melting point, it may be mentioned, is approximately 80 degrees C. or 176 degrees Fahr., while the boiling point is 218 degrees C. or 424.4 degrees Fahr. The problem was to discover to what temperature the naphthalene would have to be raised to increase the vapour pressure to such a degree that the necessary volume of air would be readily carburated, and also to decide the correct proportions of hydrocarbon and air. The laboratory experiments showed this temperature to be about 135 degrees C., and the ideal mixture to be about 1 of naphthalene vapour to 12 of air.
Reverting to the diagram, it will be observed that a proportion of the exhaust gases are drawn off to circulate round the vaporizer to raise the fuel to the requisite temperature, which, as stated above, is about 275 degrees Fahr. The exhaust is tapped at the point (A) with a pipe (B) so disposed as to form a slight baffle to assist in the defleetion.of the hot gases into B. It was ascertained that the back-pressure thsown by the silencer fitted to the ordinary exhaust pipe was sufficient to cause an adequate difference in pressure to force a certain proportion of the gases into B, and the volume of this gas is controlled by • the cock (0). It will be observed that the jacket at the bottom of the vaporizer is divided longitudinally by a division plate welded in position. This is to ensure the exhaust gases passing to the far end of the vessel, to return and finally to escape through the vent (E). Thus it will be seen that the whole of the vaporizer is equally heated. It must be explained that the exhaust gases, after fulfilling their designed duty, escape from the outlet (E), which was purposely placed within the boat to facilitate observations, but normally it would be led over the side.
The vaporizer details are of a simple character. There is a diaphragm (F) set at an angle to the ver, tical extending the full width of tilt chamber and welded at the lower end to the horizontal plate (G), which, at one end, is connected to the v-ertieaLplate (II). The horizontal plate (G) is cut to receive the lower end of a small diameter tube (J), the upper end of which extends into, and is open to, the outer air.
In the forward end of the chamber is another tube (K), .the upper open end of which is brought nearhhe top side of the tank, while the lower end is cOntinued down through its floor to a" T-piece," to which is connected the pipe coming from the ordinaryspetrol carburetter. Just above this juncture is a butterfly valve (L) to control the supply of naphthalene vapour drawn downwards to meet the -supplementary air. The operation of the vaporizer is-as follows vessel is charged with fuel, which may beseither m the form of crude powder or moulded solid, and is melted by the heat from the jacket until it reaches the. level indicated in the diagram, which is a little below the mouth of the tube (II). The suction stroke of. the
engine draws free -air to the required proportion down through the tube (J). Reaching the lower free end of the tube the incoming air tends to rise through the liquid, but by reason of the baffle (F) is forced to take the course indicated, by the arrows. Escaping from the liquid, during its passage through which it becomes saturated with naphthalene, it passes down the tube (K) past the butterfly valve (L) to the inlet manifoldlof the motor, at the inlet to which the air it is necessary to add to transform the saturated vapour into an explosive mixture is taken as described later. It will be observed that the vaporizer is of the surface type and of thvsiinplest description, while it is so designed as to ensure the air becoming earburetted to saturation at a given temperature.
As this vapour is afterwards diluted by supplementary air to form the explosive _mixture there need never be an explosive mixture in the vaporizer itself. The pipe extending upwards from the petrol carburetter, with which a junction is effected at the inlet manifold, is utilized as the supplementary air supply, this unearburetted air being drawn through the petrol carburetter, the jet of which has been closed, the
throttle acting asthe supplementary air control valve. This supplementary air should preferably be warmed, since it is inastrative that the temperature of the *gaseous mixture should be maintained right up to the inlet. As a matter of fact this precaution was not followed by the inventors during the practical experiments, the temperature of the outer air being approximately 70 degrees Fahr. It will be observed that, in this experimental model, provision is incorporated for the utilization of a thermometer, but this was in traduced merely for observing and following the temperature of the naphthalene as well as to facilitate keeping it under control. In the subsequent model it is proposed to make this control automatic. It will . be observed that, by inserting the angular diaphragm, the vaporizer is really divided into two compartments.
Trials With Motor Boat.
This was done to ensure the chamber in which the carburation of the air takes .place being sealed from that section where fuel charging is conducted, the lastnamed being fitted -with a loose lid for this purpose. While the fuel may be used in either a crude powder or solid form, the former is generally recommended, at least for starting purposes, since it melts more readily-, but the rock. form has the advantage of saving space because double the quantity can be accommodated in about one-half the space required by the
, former. An appropriate analogy is snow and ice. Both have the same melting point, but snow, owing to its looseness, will be resolved into the liquid form inore speedily than the. ace.
The engine is started up first on petrol, and run for about ten minutes, by which time the naphthalene will have been melted and raised to 275 degrees Fahr., when air .carburation will take place, as already mentioned. The inventors, in the course of the first practical test upon the River Severn, endeavoured to change over from petrol to naphthalene when the latter hacl reached a temperature of 90 degrees 0.194 degrees Fahr.—but to no purpose. It was not until the critical temperature was reached that the change-over could be readily effected, and from that moment naphthalene exclusively was used as a fuel.
Unfortunately, the investigations were somewhat marred by the butterfly valve proving to be a very poor fit and consequently refusing to act efficiently. Thus it was impossible to control the flow of the gaseous mixture, but the trouble was overcome for the occasion by opening this valve wide and adjusting the throttle of the supplementary air supply through the carburetter to meet' requirements.
The boat was run at full speed for about 1 hour. This represents the longest continuous run which Mr. Willis has been able to make on naphthalene alone, although he has used the fuel exclusively on other occasions for briefer periods.
During these brief runs, however, much interesting data was gleaned. Carburation proved an easy matter after the temperature had been carried up to 275 degrees Fahr. It was even forced up to 160 degrees 0.-314 degrees Fahr.—but increasing the temperature revealed no apparent advantage, except to set up an undue agitation of the liquefied fuel.
• Simplicity of Carburation Control.
The mixture was also varied, one test being made to ascertain the effect of a rich charge. Immediately the controlled exhaust commenced to belch black smoke composed of very finely divided particles of 'soot resembling lamp-black in composition, and such as one sets up when burning naphthalene, in such a manner as to allow the flame to play upon a. chilled surface such as a piece of metal. Alteration of the mixture was attended by the exhaust promptly reverting to a clear condition.
It must be understood that the vaporizer described as having been used in these experiments, and which is shown in the accotnpanying illustration, is purely experimental, while the investigations themselves are of a comparable character. But they have sufficed to establish one incontrovertible fact, and one whieh
038
has been convincingly demonstrated for the first time, so far as we can ascertain, at least in this country. It is possible to ruia an internal combustion engine ekelusively on naphthalene, while this hydrocarbon -offers a possible and efficient substitute for petrol. The succeeding model will also embrace a novel feature which has been borne in mind from the first. This is the reduction of the naphthalene to a molten condition in one vessel, or tank; and to allow it to flow to the carburetter proper, where the tempera, ture of the liquid will be raised to the required 'degree to ensure carburation. But, before embarking upon these further models, the inventors wish to revert once more to the laboratory, to rig up an engine on the test-bench, to collect a full range of technical data 'regarding the all-round suitability of .naphthaJena as a fuel for internal combustion engines, and to gathr details concerning power output as corn• pared with petrol, paraffin, and benzole. In view of , the stage to which the investigations have been carried, it IS to be hoped that full facilities will be granted for determining the outstanding factors, which, we may remark, are likely to lead to further far-reaching' develophaents, and to contribute very materially to our knowledge concerning the use of teavier fuels. Naphthalene offers many attractive advantages over other hydro-carbons. It is safe and clean to handle, its somewhat pungent odour before combustion being possibly its one disadvantage. 'The exhaust gases do not smell. The idea of feeding a motor with solid fuel by means of "a scoop instead of a liquid through a funnel is decidedly novel, There is no risk of, explosion as with the more volatile hydro-carbons, as no ' gas is emitted below the temperature of 275 degrees Fahr. It is not readily combustible.
Using a Waste Product.
The question arises : What is the available "amount of naphthalene and will price permit its use as a competitive fuel? The annual yield from the gas and tar works of these islands is somewhat imposing, averaging an equivalent to about 10,000,000 gallons. This ignores the possible quantity which might be derived from so treating the gas as to deprive it of its naphthalene content before _passing it into the mains— although the quantity derivable from this source is small—but the volume might certainly be further increased by securing what is sent Away in creesote oil, although the proportion in this instance may be somewhat misleading. Often creosote oil contains, as old, from 10 per cent. to 30 per cent. of naphthalene.
So far as price is concerned naphthalene may be set down as a relatively cheap fuel. At the moment it commands about 27 per ton, as compared with from 24 to 25 before the war. The specific gravity of the hydro-carbon at 61 degrees Fahr. is 1.16, compared with water at 32 degrees Fahr. The higher priced naphthalene contains from 10 to 15 per cent, of creosote oil from which it has been extracted, and the presence of which depresses the solidifying point from about 174.2 to 136 or 140 degrees Fahr.
. The final problem concerns the most advantageous fields of consumption. Obviously, in the event of the fuel being adopted for commercial vehicles, the greatest measure of success would be recorded in those instances where the conditions permit its extensive use, as, for instance, big carrying concerns such as bus companies. It should also prove an excellent fuel for agricultural tractors, inasmuch as it can be more easily transported in bulk than its liquid rivals. No special facilities or precautions are necessary for its storage. It could be dumped at any suitable Point which would be satisfactory for field work.
Are we on the verge of a new era in regard to motor fuels ? Circumstances certainly seem to point to such a concluSion. It behoves us to exploit every conceivable contribution to the home-produced fuel issue to equip us for the war after the war. Sufficient bass been done by Mr. Willis and his colleague to prove that the use of naphthalene in this field is no longer chimerical but an absolute certainty. Ultimate sue7 cess in this direction can be recorded if the exploitation'of the 'accepted fact be taken up in earnest.


























