A NEW ACCUMULATOR FOR LIGHTING.
Page 24
Page 25

If you've noticed an error in this article please click here to report it so we can fix it.
The Results of Some Tests Carried • Out with a Battery of Novel Construction.
ELECTRIC lighting is now widely used on commercial vehicles, particularly for passenger-carrying types such as buses, coaches, etc. The batteries for such service must be very soundly constructed to withstand the arduous conditions of operation, especially when the vehicle is mounted upon solid tyres which transmit more vibration than the pneumatic variety.
These arduous conditions are such that the iaverage battery, although a great improvement upon the units available some years ago, is liable to give a certain amount of trouble and has not any too long a life. Consequently, any new design is of interest, particularly when it is claimed to confer such advantages as freedom from damage through overcharging or high discharge rates, the absence of tendency for the plates to buckle, and the better adhesion of the paste.
These and other claims are made for the Tungstone battery, which was first described in the Press about two and a half months ago and which has since been tried by a number of owners of commercial vehicle fleets. We purposely deferred our description of this battery until we had satisfied ourselves by various tests that at any rate some of the claims made can he substantiated. Other advantages which this battery is said to possess can only be proved by a test more prolonged than that which we have, as yet, been able to Complete.
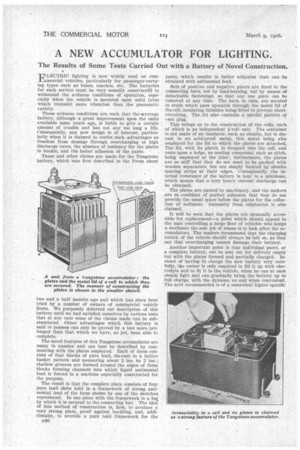
The novel features of this Tungstone accumulator are many in number and can best be described by commencing with the plates employed. Each of these consists of four blocks of pure lead, die-cast in a lattice basket pattern and measuring about 2 ins. by 2 ins.; shallow grooves are formed around the edges of these blocks forming channels into which liquid antimonial lead is forced in a machine especially constructed for the purpose.
The result is that the complete plate consists of four pure lead slabs held in a framework of strong antimonial lead of the form shown by one of the sketches reproduced. In one piece with the framework is a lug by which it is secured to the connecting bar. The idea of this method of construction is, first, to produce a very strong plate, proof against buckling, and, additionally, to provide a pure letid framework for the B40 paste, which results in better adhesion than can be obtained with antimonial lead.
Sets of positive and negative plates are fixed to the connecting bars, not by lead-burning, but by means of detachable fastenings, so that any one plate can be removed at any time. The bars, in turn, are secured to studs which pass upwards through the metal lid of the cell, insulating thimbles being fitted to prevent shortcircuiting. The lid also contains a special pattern of vent plug.
This brings us to the construction of the cells, each of which is an independent 2-volt unit. The container is not made of an insulator, such as ebonite, but is diecast in an acid-proof metal, this metal being also employed for the lid to which the plates are attached. The lid, with its plates, is dropped into the cell, and rests upon a ledge, no sealing compound, such as pitch, being employed at the joint; furthermore, the plates are so stiff that they do not need to be packed with wooden separators, but are simply located by ebonite spacing strips at their edges. Consequently, the internal resistance of the battery is kept to a minimum, which means that a very heavy current discharge can be obtained.
The plates are pasted by machinery, and the makers are so confident of perfect adhesion that they do not provide the usual space below the plates for the collection of sediment. Immunity from sulphation is also claimed.
It will be seen that the plates are unusually accessible for replacement—a point which should appeal to the man controlling a large fleet of vehicles who keeps a mechanic the sole job of whom is to look after the accumulators. The makers recommend that the charging Switch of the vehicle should always be left on, as they say that overcharging cannot damage their battery.
.Another important point is that individual parts, or a complete battery, can be sent out for delivery empty but with the plates formed and partially charged. Instead of having to charge the new battery very carefully, tbe owner is only required to fill it up with electrolyte and to fit it to the vehicle, when he can at once obtain light and can gradually bring the battery up to full charge, with the dynamo, as and when convenient. The acid recommended is of a somewhat higher specific gravity than usual-1.285—this increasing to 1.300 when the accumulator is fully charged. .
Towards the end of last year we had a 12-volt 50ampere-hour Tungstone accumulator fitted to the running-board of one of our staff ears, using it for starting the engine and for lighting. On many occasions the car was driven very considerable distances on the selfstarter and, contrary to expectations, the electrolyte did not appear to leak or creep past the lids of the cells, although these are not sealed in any way.
In January, the car was driven on the self-starter for a distance of about 201:gards on the level, and was then used to spin the engine until the battery was completely exhausted. The car was next driven for an hour over give-and-take roads with the dynamo charging at a rate of 8 amps. to 12 amps., according to the speed of the. engine. The route followed was such that, at the end of this period, the car returned to the site of the previous test, and it was then once again driven on the self-starter. On this occasion a distance of 245 yards was covered.
–Subsequently,. the accumulator was completely dismantled, and we found that the plates had not become buckled in the slightest degree. As regards the paste: this had adhered particularly well, a few flakes only having fallen out from perhaps three or four of the diamond-pattern sections of the grid in the case of the outside negative plates.
Presumably owing to the heat generated by a prolonged and heavy discharge, the thimble insulators placed round the terminal studs—where these emerge through the lids of the cells—had suffered somewhat, and there were slight signs of burning and short-circuiting in two or three instances at the edges of the metal cell containers. Considering the treatment to which it had been subjected, it may be said that the accumulator withstood the test remarkably well. It was then recharged on the bench, following which it was cOupled to a starter-motor fitted with a brake. Teats under these conditions showed it to be extremely powerful, and also showed that no real damage had occurred in the course of the extraordinarily severe test to which it had been subjected.
Our only criticism is that some leakage of current is liable to occur owing to the fact that die-cast metal containers are employed, and whilst we realize that the use of metal enables the heat to be dissipated more readily, we think that an insulated container is in some ways less likely to kive trouble. The construction of the plates themselves seems admirable and efficient The makers of this battery are the Tungstone Accumulator Co., Ltd., 3, St. Bride's House, Salisbury Square, London, E.C.4.




























