Simple Language about Steel Composition.
Page 3

Page 4

Page 5

Page 6
If you've noticed an error in this article please click here to report it so we can fix it.
The subject of steels for motor-vehicle construction is one of absorbing interest to many of our readers, and several articles dealing with their characteristics, uses, etc., have appeared in the pages of this journal during recent years, Any fresh view of the subject, or new method of treatment is sure to be of interest to most practical men. Mr. E. F. Lake, in recent issues of " The Automobile," of New York, has been dealing with the composition of steels, and the effect which carbon has upon the processes of annealing and hardening, in a manner which should readily be understood by most shopmen, who have no time to give to the questions involved in all their branches, but who desire to become acquainted with the principles that underlie intelligent handling of steel in the shops. The substance of Mr. Lake's articles has been extracted, and is given herewith for the benefit of our numerous readers who are interested in the subject.
The Effect of Carbon on Tensile Strength.
Carbon is the most important of all the elements or materials that are used in the manufacture of steel products or that are naturally present in the ores or finished metal, and, in dividing steel products into different grades, the division is based on the carbon content in the vast majority of cases. Other elements may form the basis in various alloys, but most of these are sub-divided according to the percentage of carbon. It unites with pure iron in all proportions up to 4.5 per cent., and by adding a high percentage of manganese to the metal the carbon content can be raised to 7 or 8 per cent.
It has been demonstrated that each increase of 0.01 per cent, of carbon increases the strength of pure iron anywhere from 750 to 1,150 lb. per sq. in., until about 0.90 per cent. has been reached, after which the carbon begins to separate into graphitic carbon. When 2.00 per cent, of carbon is reached, the graphitic carbon Ls very pronounced, and beyond this point the metal is usually in the form of cast iron. The percentage of carbon that produces these increases in strength is out of all proportion to the mass of metal with which it is combined. This is one of the wonders in metallurgy, as no other combination gives anywhere near such results.
Manganese and nickel take practically the same effect on iron as does carbon, but nowhere near in the same proportions. Thus the results obtained with 1.00 per cent, of carbon could not be obtained with less than 7.25 per cent, of manganese, or 17.55 per cent, of nickel. All three of the cause the metal to undergo a structural change from pearlite, that includes the sorbitic, to martensite, that includes the troositic, and then to the polyhedral structure, and with none of them is a special carbide formed. Chromium has an analogous effect, but not as complete as a double carbide of iron and chromium forms, and this is not maintained in solution in the iron without tempering. The desired percentage of carbon is given to steel in different ways in the different steel-making processes used. The Bessemer process burns out all of the silicon first, then the manganese, and next the carbon, after which the desired amount of carbon is recharged before pouring the metal into ingots. In the open-hearth" process the excess carbon, present in the pig iron, is oxidized out by boiling the metal until the carbon has been reduced to the desired percentage. In the crucible process, muck-bar (a form of wrought iron that contains 0.10 per cent. of carbon or less) is used for the base or stock, and when this is charged in the crucible charcoal is added to give the metal the desired carbon percentage. Still another well-known method is used to add carbon to steel products, and that is the carbonizing process. With this method, a low-carbon metal is heated to about 1,650 degrees F. and then caused to absorb carbon from the outside by packing it, in sums carbonaceous material, such as bone and charcoal, or submitting it to the action of a carbonaceous :2;as in a sealed chamber or retort.
Proportion of Carbon in Iron and Steel.
When the carbon content of the iron products is below 0.10 per neut., the metal is called wrought iron. From 0.10 to 0.30 per cent. it is called soft or machinery steel and cannot be hardened enough to prevent a file from cutting it. When the carbon content is from 0.30 to 2.00 per cent., the steel can be hardened so as to cut other steels or metals, and is then called tool, half-hard, hard, or high-carbon steel. Exceptions might be made to the term hard steel, as it is possible to make steels yery hard with manganese, tungsten or chromium, and still have a low percentage of carbon. Each increase in the percentage of carbon increases the hardness and brittleness of steel, and makes it more liable to fracture when cold, or when heated suddenly. The tenacity shows a pronounced increase up to a carbon content of 0.90 per cent. and a slight increase from there to 1.20 per cent., after which it rapidly decreases. The ductility keeps do. creasing from the minute carbon begins to show in the metal. It decreases rapidly when the carbon content is raised from 0.30 to 0.90 per cent., but before and after this the decrease is comparatively slow.
The difference in strength produced by each increase of 0.01 per cent, of carbon should show the necessity of drawing the contract specifications as close as possible for steels used in automobile construction. The degree of hardness also increases with each increase in the percentage of carbon; the hardening properties are likewise increased. As a general rule, hardness and strength increase simultaneously in all metals, up to a certain point. Hence, specifications that allow from 0.25 to 0.35 per cent, of carbon would cover a range that would give two entirely different products. A 0.25 per cent. carbon steel cannot be hardened in the ordinary acceptance of the term, while a 0.35 per cent. carbon steel can be hardened so that a file will not cut, it and it can be used as a tool for cutting metals. This 0.10 per cent. variation would give a difference in strength of from 7,500 to 11,500 lb. per sq. in. In designing a motor vehicle, in which lightness is a factor, this amount of variation in the strength of metal would hardly be allowable, and might cause trouble. An allowance of 0.10 per cent, variation in the specifications for carbon, when the percentage is as low as in the above-mentioned steel, would make it look as though these specifications were drawn in the interest of the steel makers and the needs of the steel users were ignored. A variation of 0.05 per cent, is all that is needed in making steels of this grade and is as much of an allowance as is asked for by steel makers who turn out a good prnduct.
Carbon as it Relates to Annealing.
In the annealed steels it is possible very closely to determine the carbon content by a microscopical examination. The microscope shows that steel is not a simple substance, but is a conglomeration of the crystals of different substances. Ferrite, pearlite and cementite can be seen in the ordinary carbon steels that, have been thoroughly annealed. When we know the properties of these substances and the proportions in which they exist in steel we can determine many of its characteristics. In actual practice, however, some allowances have to be made for the impurities that might be present in the metal or for other ingredients or alloying materials.
If we take steels with varying carbon contents and give the different samples a perfectly smooth polish and etch this surface with picric or hydrofluoric acid, tincture of iodine or other etching materials, we can clearly see these different constituents under the microscope. Starting with carbonless iron that is pure, we find ferrite to be the only constituent. When we begin to add carbon, however, pearlite appears and gradually increases until 0.85 per cent. of carbon has been reached. At this percentage the pearlite has completely driven out the ferrite and is the only constituent that shows. From this point, on cementite begins to appear, and if it were commercially possible to make steel that contained 6.60 per cent, of combined carbon without any graphite carbon being present the cementite would obliterate the other two constituents and be the only one seen in thoroughly annealed steel.
The ferrite crystals, in theory, contain no carbon or other impurities. The properties of ferrite are such that the greater its percentage the greater will be the metal's softness and ductility, and the higher will be its electric conductivity and magnetic strength. When steels below 0.85 per cent. of carbon are polished and etchid with. picric acid, their surfaces will show white with dark masses. The white is ferrite and the dark masses are pearlite; consequently these dark masses are the more numerous the closer we approach the carbon content of 0.85 per cent.
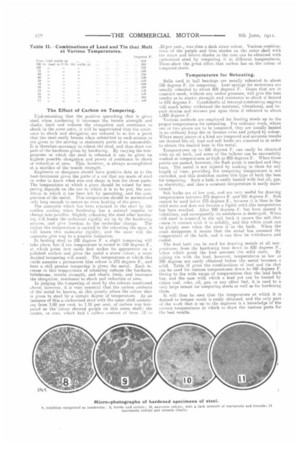
In Fig. I will be seen a micro-photograph of a steel so low in carbon that it might be classed with the irons. As will be seen, the surface is almost entirely covered with the white ferrite. As the carbon content rises pearlite begins to show in the form of separated pearlite islands in a ferrite matrix, as shown in Fig. 2. Further increases in the carbon content make these dark masses spread until they become connected and appear in streaks similar to the formation illustrated by Fig. 3. When the carbon content has reached 0.85 per cent. these dark patches nearly cover the entire surface, and the metal has the appearance of that shown in Fig. 4.
The pearlite is an intimate mixture of ferrite and cementite the latter of which begins to appear after the carbon content has passed the point represented by 0.85 per cent. It, consists of 32 parts of ferrite to 5 of cementite, which gives it the appearance of mother of pearl, from which it derives its name. It exists either as alternate plates of ferrite and cementite, or in a granular formation composed of intermingling grains of ferrite and cementite. When the pearlite is predominant the metal has the finest crystalline structure of any of the carbon steels, and it is the strongest. By proper heat treating this metal can be given a greater degree of combined hardness and toughness than any of the other steels. When thoroughly annealed a rod inch in diameter can be tied into knots when cold without its cracking. This well indicates the combined toughness, ductility and strength to be found in this grade of steel.
Cementite is a carbide of iron that is expressed by the formula Fe5 C. Practically all of the carbon is present in this form, and it usually crystallizes in thin flat plates. It is the second constituent of importance in steel, and of itself contains 6.60 per cent. of carbon, which is about one-fifteenth of its mass. Its more important characteristics are its great hardness, brittleness, and consequently lack of strength. It first takes the form of a continuous skeleton framework, as shown in the pearlite matrix in Fig. 5. As the carbon content increases this skeleton develops into a mass of patches that, are connected with one another, as shown in Fig. 6, where its area is about equal to that of the pearlite.
The structure indicated by these micro-photographs is only seen when the steel has been thoroughly annealed. When the metal is heated to higher temperatures and quenched, as in hardening, these constituents are altered. If we take a steel consisting entirely of pearlite and heat it to 1,350 degrees F. the pearlite disappears and polyhedral crystals that form a homogeneous white substance called austenite make their appearance. When quenched at different temperatures martonsite, sorbite and troosite become constituents of the metal. These will be discussed later.
To form definite conclusions on the characteristics of the metal from these structures it would be necessary to know the chemical composition of the metal, as some of the ingredients or alloying materials might alter their appearance. As an instance, Fig. 7 shows a chrome-vanadium steel that is in the same annealed state as the carbon steel shown in Fig. 6. As will be seen, the cementite and pearlite are practically in the same proportions, but the much finer grain shown in the chrome-vanadium steel might be misleading. The chromium might also change the result given by the carbon content. The etching material is important, as one will make the ferrite, pearlite or cementite show black, and another will make them appear white.
On the Hardening of Steel.
As there are so many parts in the make-up of an automobile that give much better results when made of steel that has been hardened, it will probably be well to go into the principles of hardening steel and trace their effects. The hardening that alters and improves the static strength and dynamic qualities of steel to obtain better results for motorcar parts is the only kind we will treat of in this article. While hardening steel for cutting tools is very important, it has no bearing on this subject. In the latter case the carbon content must be above 0.30 per cent., or the metal cannot be made to assume a hardness that will give it a good cutting edge. In the first case, however, a greater tensile strength, elastic limit, etc., or better wearing qualities may be obtained from steels of any carbon content whether above or below 0.30 per cent. This is more clearly shown in Table I, in which (page 355) the chemical composition and static strengths are given of six steels with different carbon contents, in both the annealed and hardened condition.
To give steel any degree of hardness it is necessary, in theory, to raise its temperature to the highest point of transformation, hold it there long enough for the grain structure of the entire mass to assume its new form, and then instantaneously cool the metal to atmospheric temperature. This will hold the new-born grain structure in the form it has assumed. In practice it is necessary to raise the temperature a few degrees above the highest recalescence point to allow for
that lost in passing the steel from the furnace to the quenching bath, and also allow for the slow cooling when in the quenching bath.
The principle on which the hardening property is based is that a grain of iron carbide and one of pure iron lie side by eide disunited. Annealing has caused this separation, which is only microscopical, to take place between them, but they still lie so close together that. they can easily be united. Heating the metal to just above the highest recalescence point causes them to combine, and this unity can be made permanent by suddenly cooling the steel from this high temperature. The union causes the steel to show a substance of a Lamy nature when microseopically examined. This is a new constituent that has been born. It is called martensite and hardenite.
alartensite is discovered from lines intersecting each other in the direction of the sides of an equilateral triangle, as shown in Fig. 8. It is the principal constituent of all ordinary hardened steels with a carbon content above 0.16 per cent., and tempered steels owe it their quality of hardness. A 0.85 per cent, carbon steel heated to 1,400 degrees F. and suddenly quenched will show the martensite very pronounced. It is so hard that a needle will not scratch it. When more. than 0.85 per cent, of carbon is present the martensite is said to. he saturated, and shows slightly different under the microscope. In that condition it is often called hardenite.
When the rate of cooling is not as rapid as that produced by quenching, but is still much faster than the slow cooling of annealing, another constituent, called sorbite, is produced. This can be obtained by quenching immediately below, or just at the end of cooling through the critical range, by cooling quickly through the critical range without actual quenching, or by rapidly cooling the steel and then reheating it to about 1,100 degrees F.
Sorbite is not clearly defined in micro-photographs, but Fig. 9 shows it fairly well in the 'presence of ferrite. Sorbite is a constituent between cementite and pearlite, and chiefly differs by the crystals of these segregating and not quite per
fectly developing. The sorbite structure is finer than the pearlite, and it is considered the extreme opposite of the crystalline structure. In hardening steel it is considered as the transition from cementite to martensite, and is necessary in steels that must resist wear and erosion. It is possible to produce a natural sorbitic formation by the addition of certain alloying elements to steel.
Steels that contain over 1.10 per cent. of carbon and are suddenly cooled from a temperature of 2,000 degrees F. will, in addition to martensite, show a constituent that may be dietinguished from it, by a different colour. After etching with a 10 per cent. solution of hydrofluoric acid or nitrate of ammonia it, will show white. This constituent is softer than martensite, and is easily scratched with a needle.. It is a conglomeration of ferrite and cementite, and has been named atustenite.
It is difficult to preserve austenite throughout the whole structure of the steel, If the metal is quenched in a bath below the freezing point, or by other means that will cool it rapidly, it will show more prominently. Tempering the metal afterwards loosens the austenite, and thus it is difficult to find in steels that have been heatareated for commercial purposes. It is not of much practical use, owing to the high temperature at which it is obtained. It may be seen in Fig. 10, where it is shown in the form of white portions among the black martensite and troosite. formations. When steel is quenched at, or just above, its highest recaleee cence point in a bath of little activity, such as oil, a constituent called troosite is obtained. Trooeite shows jet black if the metal is polished and etched with picric acid. If etched with tincture of iodine it will show white. It may also be obtained by hardening steel in the usual way and then tempering it. It is softer than martensite, and can be scratched with a needle. It holds some ferrite, austenite, and cenientite, or a combination of these. It is readily found in most. tempered steels, as it is a product of the usual tempering operations. It shades gradually into the sorbite, but is very sharp in its divisions from martensite.
By submitting steel to different heat-treatments the conetituente can be changed from pearlite to matteasite or hardenite, sorbite, austanite and troesite, and back again through these different stages without in any way changing its chemical composition. By microscopical examination it is possible closely to judge the heat treatment to which steel has been subjected. The different constituents indicate the change made in the constitution of the steel, that is the static strengths and dynamic properties that have been altered. It. is here that the automobile engineer or designer can practically apply this knowledge, as with it he can design different parts, whether they be moving or stationary, so that the best results may be obtained with the smallest quantity of material. The constitution of a given steel is not the same in the hardened that it is in the normal state. In the annealed or normal steel the carbon is in a free state, while in hardened steel it is in a state of solution, which we may call martensite. This contains more or less carbon, according to the original carbon content. The mechanical properties depend principally upon the carbon content, and they are changed slowly by an increase in carbon.
The automobile builder needs to find a hardening process that will make a steel as homogeneous as poesible. In a steel containing 0.85 per cent, of carbon this is easily obtained by passing the upper rece,leseence 'mint before the material is plunged into the quenching bath.
In examining steels that have been quenched above this point, it has been found that the higher temperatures to which the metal is raised the coarser will -be the martensite, and the lines will be more visible. If the temperature is raised a few hundred degrees, and the metal he quenched in a very told bath, auetenite makes its appearance. As the martensite coarsens, the tensile strength and elongation are proportionately reduced until they become nil. The reduction of area also lessens. It has been demonstrated that 40 degrees above the highest point of recaleseence is the extreme limit to which steel can be raised to obtain the best results in hardening as well as in annealing.
'Co sum up, we might say, all steels may be hardened, but when the carbon content is over 0.30 per cent. the effect is more pronounced ; hardening increases the tensile strength and elastic limit and reduces the elongation, the effect being greater the greater the carbon content; quenching at the proper temperature gives the metal greater homogeneity and adds to its power of resistance to shock, especially low carbon steels; the temperature to which steel should be raised for hardening should not be more than 40 degrees above its highest transformation point, as beyond that it, no longer has the same qualities. The use of gas-heated furnaces is desirable to ensure uniform working.
The Effect of Carbon on Tempering.
Understanding that the peaitive quenching that is given steel when naraening it increases the tensile strength and elastic limit and reduces the elongation and resistance to shock in the same ratio, it will be appreciated that the resistance to shock and elongation are reduced to so low a point that the steel easily breaks when submitted to such 'strains as are given to the moving or stationary parts of an automobile. It is therefore necessary to reheat the steel, and thus draw out part of the hardness given by hardening. By varying the temperature at which the steel is reheated it can be given es highest possible elongation and power of resistance to shock or reduction of area. This, however, is always accomplished at a sacrifice of the tensile strength. Engineers or designers should have positive data as to the heat-treatment given the parts of a car that are made of steel in order to know what size and shape is best for these parts. The temperature at which a piece should be raised for tempering depends on the use to which it is to be put, the condition in which it has been left by quenching, and the composition of the metal. This temperature should be maintained ante long enough to assure an even heating of the piece. The austenite which has been retained in the steel by the sudden cooling when hardening has a natural impulse to change into pearlite. Slightly reheating the steel after hardening will lessen the molecular rigidity set up by the hardening process, and give freedom to the molecular change. The nigher the temperature is carried in the reheating the more it will lessen this molecular rigidity, and the more will the austeeite give way to a pearlite formation.
In heating steel to 150 degrees F. a slight tempering will take place, but if the temperature is raised to 450 degrees F., at which pnint iron oxide first makes its appearance on a polished surface and gives the metal a straw colour, a more decided tempering will result. The temperature at which this oxide assumes a permanent. blue colour is 575 degrees F., and here a still greater tempering is given the metal. Each increase in this tempeseture of reheating reduces the hardness, hrittlenees, tensile strength, and elastic limit, and increases the elongation, resistance to shock and reduction of area.
In judging the tempering of steel be the colours mentioned above, however, it is very essential tbat the carbon contents of the metal be known, as this greatly alters the colour that i$ given to steel by a certain degree of temperature. As an instance of this a carbonized steel with the outer shell containing from 1.00 per cent. to 1.10 per cent, of carbon was tempered so the' colour showed purple on this outer shell ; the centre, or core, which had a carbon content cif from .15 to
.30 per cent., was then a dark straw colour. Various combinebona of the purple and blue shades on the outer shell with toe straw and brown shades in the cure can be obtained with carbueized steel by tempering it at different temperatures. These show the great effect .that carbon has on the colour of tempered steels.
Temperatures for Reheating.
Balls used in ball bearings are usually reheated to about 350 degrees F. for tempering. Leaf springs for motorcars are usually reheated to about 800 degrees F. Gears that are in constant mesh, without any undue pressure, will give the best. results as to elastic strength and resistance to shuck if heated to 675 degrees F. Crankshafts of internal-combustion engines will much better withstand the torsional, vibrational, and impact strains and stresses put upon them if reheated to about 1,000 degrees F. Various methods are employed for heating steels up to the proper temperatures for tempering. For ordinary work, where one or two pieces are to be tempered, they are usually heated in an ordinary forge fire or furnace oven and judged. by colour. Where many pieces of a kind are tempered and accurate results are desired the oil, lead and salt baths are resorted to in order to obtain the desired heat in the metal.
Temperatures up to 600 degrees F. can easily be obtained with an oil bath, and some of the billows can be successfully worked at temperatures as high as 800 degrees F. When these points are passed, however, the flash point is reached and they burn. The metal is not injured by soaking in them for any length of time, providing the tempering temperature is not exceeded, and this doubtless makes this type of bath the best for tempering. Such a bath is easily heated with fuel oil, gas, or electricity, and thus a constant temperature is easily maintained.
Salt baths are of low cost, and are very useful for drawing temperatures between 575 degrees F. and 900 degrees F. Salt cannot be used below 575 degrees F., because it is then in the solid state and does not become a liquid until this temperature has been reached. After 900 degrees F. has been passed it volatilizes, and consequently its usefulness is destroyed. When cold steel is inserted in the salt bath it causes the salt that comes in contact with it to solidify, and this white crust can be plainly seen when the piece is in the bath. When the crust disappears it means that the metal has assumed the temperature of the bath, and it can then be withdrawn and cooled.
The lead bath eau be used for drawing metals at all temperatures, from the hardening heat down to 620 degrees F.,
below which point the lead assumes the solid state. By mixing tin with the lead, however, temperatures as low as 300 degrees are easily obtained before the metal becomes a solid. Table II gives the combinations of lead and tin that can be used for various temperatures down to 450 degrees. F. Owing to the wide range of temperatures that the lead bath has, and the ease with which a lead pat can he heated by either coal, coke, oil, gas, or any other fuel, it is used to a very large extent for tempering steels as well as for hardening them.
It will thus be seen that the temperature at which it is desired to temper steels is easily obtained, and the only part of the work that is up to the engineer is a knowledge of the correct temperatures at which to draw the various parts for the beet results.






























