I . a Tell-tale to Fig. 2.
Page 32
Page 33

If you've noticed an error in this article please click here to report it so we can fix it.
ENGINE EFFICIENCY
nNE of the chief factors governing the power developed by both petrol and oil engines is the manner in
which combustion i s achieved. If a high mean effective pressure •is to be obtained, the combustion must be rapid and complete, the composition of the ex.haust gas being an excellent guide as to the extent to which this ideal is realized.
Both petrol and oil fuel are hydrocarbons, consisting of approximately 85 per cent. carbon and the remainder mainly hydrogen. When combustion is complete, the carbon unites with the oxygen of the air to form carbon dioxide, and oxygen combines with the hydrogen to form water or steam at the temperature of combustion. Those familiar with chemical formulm will recognize these reactions in the equations C + 02= CO, and 2H2 +02=2H2O.
Nitrogen Dilutes Oxygen
Nitrogen, which forms about four-fifths of the air by volume, takes no part in these reactions, merely acting as a diluent of the oxygen.
Theoretically, there should be the three gases, carbon dioxide, steam and nitrogen, in the exhaust, all of which are colourless and free from smell. This assumes that there is sufficient oxygen present to unite with all the particles of carbon and hydrogen.
In practice, perfect mixing of the oxygen and fuel particles is not possible, so that an excess of air must be provided, particularly in the case of oil engines. Should there be a lack of oxygen, such as when starting on a rich petrol-air mixture, the carbon will not be completely oxidized and carbon monoxide will be formed. In addition to being dangerous if inhaled, this gas also A30
represents a direct loss of heat generated, so that in this connection alone, a rich mixture is undesirable, except for the purpose of starting.
An average hydrocarbon fuel requires about 14.5 lb. of air for complete combustion. On a petrol engine this represents a normal mixture ratio, the average carburetter giving a range between approximately 8 to 1 for starting and 16 to 1 for economical running under cruising conditions.
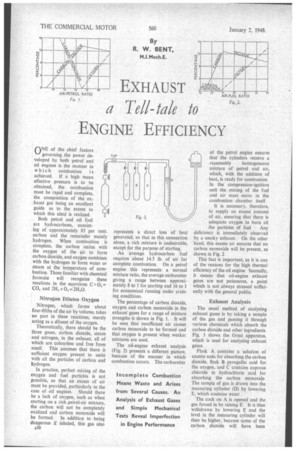
The percentage of carbon dioxide, oxygen and carbon monoxide in the exhaust gases for a range of mixture strengths is shown in Fig. I. It will be seen that insufficient air causes carbon monoxide to be formed and that oxygen is present when weaker mixtures are used.
The oil-engine exhaust analysis (Fig. 2) presents a different picture, because of the manner in which combustion occurs. The carburetter of the petrol engine ensures that the cylinders receive a reasonably homogeneous mixture of petrol and air, which, with the addition of heat, is ready for combustion. In the compression-ignition unit the mixing of the fuel and air must occur in the combustion chamber itself.
It is necessary, therefore, to supply an excess amount of air, ensuring that there is adequate oxygen to burn all the particles of fuel Any deficiency is immediately observed by a smoky exhaust. On the other hand, this excess air ensures that no carbon monoxide will be present, as shown in Fig. 2.
This fact is important, as it is one of the reasons for the high thermal efficiency of the oil engine. Secondly, it means that oil-engine exhaust gases are not poisonous, a point which is not always stressed sufficiently with the general public.
Exhaust Analysis
The usual method of analysing exhaust gases is by taking a sample of the gas and passing it through various chemicals which absorb the carbon dioxide and other ingredients. Fig. 3 shows the Orsat apparatus, which is used for analysing exhaust gases.
Flask A contains a solution of caustic soda for absorbing the carbon dioxide, flask B pyrogallic acid for the oxygen, and C contains cuprous chloride in hydrochloric 'acid for absorbing the carbon monoxide The sample of gas is drawn into the measuring cylinder (D) by lowering E, which contains water
The cock on A is opened and the gas forced in by raising E. It is then withdrawn by lowering E and the level in the measuring cylinder will then be higher, because some of the carbon dioxide will have been absorbed. The process is repeated for flasks B and C, thus obtaining the percentage of . carbon dioxide, oxygen and carbon monoxide, the balance being assumed to be nitrogen.
It is obvious that factors other than the quantity of excess air will affect the efficiency of combustion. For example, it has been assumed that the fuel consists simply of hydrogen and carbon, but it will also contain small percentages of other substances. A small amount of sulphur may be present, and this will appear in the exhaust gases, giving them a characteristic and unpleasant smell.
Controlling the Air Flow
Turbulence is important in petrol engines and vitally so in oil engines. Turbulence is a controlled swirl, enabling the oxygen to seek out each particle of fuel. Thus on oil engines there are shrouded inlet valves to give a swirl to the induced air and specially recessed pistons to ensure both vertical and horizontal rotation of the air stream.
On petrol engines, ignition plays an important part in ensuring complete combustion. Thus the correct position and type of sparking plug and the time at which ignition occurs will all help in securing corn
bustion that is both rapid and free from detonation Visual observation of the exhaust is helpful in indicating what is taking place in the cylinder Thus, on a petrol engine it is easy to detect the difference between a rich mixture. causing a blackish exhaust, and one where oil is passing up the pistons and emitting blue smoke.
Oil engines will smoke when the nozzles are not functioning properly, or if the fuel pump be metering an unequal quantity of fuel to each cylinder There is no doubt that some operators do not realize the importance of regular nozzle cleaning in order to ensure a smoke-free exhaust Apart from the bad effect on public opinion of a vehicle discharging a cloud of smoke, it should be noted that fuel is wasted.
The position of the exhaust outlet on vehicles carrying foodstuffs, etc., which may be contaminated by exhaust fumes, is important_ On public service vehicles it is obligatory that the pipe shall terminate•on the off side of the vehicle, but in some goods chassis it could well be extended to the rear of the vehicle. To secure maximum dissipation of the fumes, the pipe should finish in a position where it meets the maximum air velocity
When an exhaust joint is blown, the resultant hissing noise will indi cate the seat of the trouble If, when a new joint has been fitted, it blows again in a short time, the faces of the manifold should he checked Sometimes the constant heating and cooling will distort the manifold Attention with a file and a face plate should soon remedy this trouble Fumes issuing from the crankcase breather may also cause trouble Usually these emerge under the bonnet, to be dispersed by the air stream from the fan The can. however, find their way into the cab, especially if the piston rings permit a certain amount of blow-by Dispersing Crankcase Fumes Some makers fit a separate pipe to the crankcase, leading it through the engine tray, so that it discharges direct to the atmosphere This arrangement prevents fumes from passing into the cab and probably improves the crankcase ventilation.
An alternative on petrol engines is to connect the breather to the car
buretter inlet This system has to be used with discretion, as in one instance it was found that not only fumes but most of the oil mist was being drawn from the crankcase. This mist plays an important part in lubricating components not directly force-fed.












































































