The Equipment of Case-hardening Rooms.
Page 2

Page 3

If you've noticed an error in this article please click here to report it so we can fix it.
The Metallic-salt-bath Type of Hardening Furnace.
(Continued from page 338.) In our last issue we described a few typical oil-fired and ga .3-fi red furnaces, such as are now obtainable from the makers whose names we have quoted, but, before proceeding to describe those furnaces in which the parts to be treated are p■aced in a bath of molten metal or metallic salts, we would draw our readers' attention to the illustration of a large, gas-fired, double-ended furnace which is made by Fletcher, Russell and Company, Limited, of Warrington : this company is closely associated with the production of all kinds of muffles and furnaces which are designed to consume liquid fuel, coal gas, or producer gas. The furnace shown on this page has been designed to do the work of one of the usual coke-fired furnaces, such as we described in our issue of the 28th ultimo, and, as is usual with that type of furnace, the parts that are to be hardened must be closely packed in boxes. The view shows the furnace with part of one of its side walls cut away, in order to expose to view the two boxes which are contained within the oven. The double doors allow of the furnace's being charged, or drawn, from either end. The boxes are completely enveloped in the flames or the hot gas from the row of burners beneath the bottom slab.
Liquid Hardening Furnaces.
Although, in the case of muffle ovens, the steel does not come into contact with the products of combustion, it is not possible entirely to keep oft the air while the hot steel is being transferred from the furnace to the quenching bath. With a view to obviating this difficulty, many attempts to heat the parts while submerged in a liquid bath have been made, and these have been attended with varying degrees of success, according to the design of the furnace, or the selection of the material for the bath. The difficulties have been sufficient to prevent any general use being made of the method. The advantages which attend the employment of such a furnace are very great, and chief amongst these are : freedom from oxidation, because, during the period of heating the metal is submerged in the liquid and is there protected from the air ; the metal, while being transferred from the bath to the quenching tank, is coated with a thin pro-tecting film of the material of the bath; the temperature of the liquid may be accurately regulated, and cannot vary in a few minutes, as does the atmosphere of a furnace; and any article which is immersed for a sufficiently long period must become uniformly heated to the temperature of the bath, whilst the sharpest corner cannot be overheated so long as the liquid itself is kept at the desired temperature. The materials which were most usually employed during the early days of liquid hardening furnaces were lead, antimony, and cyanate of potassium, but the impurities which these materials contain, such as sulphur, are likely to he set free, and they will then pass over and become absorbed by the steel. The poisonous vapours which are generated when lead and cyanate of potassium are heated are very injurious to the men who have to operate the furnaces. Further objections are : molten lead does not circulate very freely, and the temperature of the bath may, therefore, in a short lime, vary considerably in different parts of the bath ; even when the surface of the lead is covered with charcoal, as it should be, there is generally trouble with the formation of dross, and the high specific gravity of the lead (ii) is so objectionable that its, use is prohibited for many kinds of work, for not only is a great weight of lead required, but steel parts, on account of their lower specific gravity, must be forced and anchored down, or they will float on the surface and not be heated uniformly.
The most perfect form of furnace is one in which a bath of metallic salts is employed. These give off but little vapour, and any desired temperature up to 2,370 degrees Fahrenheit can be obtained at the will of the operator. The following mixtures of metallic salts, have been found to give good results :—For compara_ tively low temperatures, a mixture of three parts of chloride of barium to two Parts of chloride of potassium, the melting point of which
mixture is about 7,200 degrees Fahrenheit; for temperatures above that, but below 1,830 degrees Fahrenheit, use a mixture of sodium hydrate (8 per cent.), sodium chloride (91 per cent.), potassium nitrate (i per cent.), and potassium chromate (.i per cent.); whilst, for temperatures above 1,830 degrees Fahrenheit, it is advisable to use pure crystalline chloride of barium. These materials are reduced to a molten state by the heat from gas or oil burners, or by means of the electric current, and the parts that are to be hardened are then placed on suitable trays which can be lowered into, or
raised from t h e liquid. The " melt " thus circulates freely over every part with in the containing pot, and the temperature of the work and of the pyrometer must be the same after the parts have been immersed for a length of time sufficient to ensure that they have been thoroughly heated throughout.
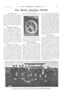
The most successful furnace of this type is that which we illustrate on this page. This furnace is fitted with two sets of burners ; one set, coinprising four burners, is for use with an air blast, and the other set consists of a number of Bunsen burners, which are used without the aid of an air blast. Gas is fed to the burners, through the pipes (G and H), and air through the pipe (J). It is not intended that the two sets of burners shall be used at the same time ; the top four burners, with the air blast, are used for rapidly melting the salts, but for accurately regulating and maintaining the temperature the Bunsen burners are more suitable. If an air blast is not available, the furnace can be worked with the Bunsen burners only, but a longer time must be allowed for the first melting. Taps are fitted to each of the connections (G, H, and .1), and these are provided with large graduated quadrants for regulating the supplies of air or gas to the burners. The outer casing of the furnace is of wrought iron, lined with fire brick, and it is provided with a counter-balanced door in front, and a suitable flue for the discharge of the burnt gases at the back. The whole construction stands on a cast-iron trough. The iron or steel pot (B), which contains the " melt," stands on a fire-brick pedestal (A) in the centre of the trough. The pot is 14 inches in diameter, and 20 inches deep inside. In the centre of the furnace, there is a counter-balanced tray (C), which may be raised or lowered as is required. This tray is carried by two rods (D, D), which pass through holes in the top cover, and they are joined together by a yoke piece (F), into which a hook is fitted. The pyrometer (E) hangs through a hole in the back of the tray. This furnace is made by Mr. S. N. Brayshaw, of 2, Mulberry Street, Hultne, Manchester, and another furnace of this type is made by Fletcher, Russell and Company, Limited, of Warrington.
Colonel Crompton, C.B., R.E., in his researches upon the heat treatment of steel, and, later, in his work in connection with the preparing of standard thread gauges for the British Engineering Standards Committee, used a gas-fired
Interior arrangement of Brayshaw's salt-bath furnace. furnace of this type, in conjunction with an ingenious device for lowering into the bath the part to be heated. This consisted of a long vertical spindle, on which a quick-pitch thread was cut, and the work was gripped in a chuck at the lower end of the spindle; as the work was lowered into the bath, it was given a rotary motion by reason of the spindle's passing through a fixed nut. The two principal advantages of this arrangement are : distortion, due to the weight of the work, is avoided; and, the rotary motion prevents the accumulation of air bubbles on the surface of the work. Col. Crompton employed a lead bath, on the surface of which a layer of liquid salt floated. As the cold work passed through the top-layer of fluid, a film of the salt solidified on to the work, and this protecting film prevented the lead from actually coming into contact with the work until the temperature of the latter had risen sufficiently to melt off the salt.
Electrically-heated "Liquid" Furnaces.
Metallic salts do not conduct current when they are in the cold state, but they are excellent conductors when in the hot, liquid condition. In order to reduce the salts to a molten state, therefore, a powerful electric arc must be employed, or, another way of starting such a furnace is to connect the two electrodes within the pot by means of a rod of carbon ; this rod, when it becomes white hot, melts the salt.
Advocates of this kind of furnace claim that it is the only one in which the heat is uniform throughout the bath, but the differences between the results obtained with a gas-fired furnace, and those from an electrically-heated bath, are too small to be seriously considered.
With either of these types, the crucible which forms the inner part of the pot need not be replaced should it crack in use : the liquid salt will fill in the cracks, and will solidify there. The consumption of the metallic salts is very small, and it has been found that when working at a temperature of 2,370 degrees Fahrenheit, only two pounds of salt have I) be added for each to hours' working; the cost of maintaining a salt-bath furnace is not, therefore, very large.